Oxygen has helped us understand how life on Earth has evolved by offering crucial hints about geological processes. The non-metallic oxygen atom, which is located in group 16 and period 2 of the periodic table, is unusually reactive and forms compounds with nearly all other elements. It plays a role in several processes that create or protect planets, create living things (via DNA, proteins, lipids, and carbohydrates), and performs metabolic functions (photosynthesis and respiration). It is present in all of the Earth’s surface water, biological reservoirs, atmosphere, mantle, and reservoirs that transmit oxygen.
History of Oxygen
Oxygen has a long and fascinating history dating back to Carl Wilhelm Scheele’s first discovery of it in Uppsala in 1773 and Joseph Priestley’s publishing of it two years later. By using Greek roots (oxy and -genes) to refer to oxygen as the “creator of acids” since he believed all acids included oxygen, Lavoisier performed a significant contribution to the discovery of the mechanism by which oxidation or combustion occurs. The history of civilization has seen oxygen play a wide range of roles, including energy generation (hydrologically or as a general fuel oxidant), agriculture, the creation of textiles and ceramics, as well as the manufacture of numerous pharmaceuticals.
- In 1772, a Swedish scientist named Carl Wilhelm Scheele isolated oxygen by heating a variety of chemicals, including potassium nitrate, mercuric oxide, and many more.
- Priestley, an English scientist, independently discovered oxygen in 1774 by the thermal breakdown of mercuric oxide and published his discoveries the same year, three years before Scheele’s publication.
- With remarkable insight, French chemist Antoine-Laurent Lavoisier in 1775–80 interpreted the role of oxygen in respiration and combustion, rejecting the phlogiston theory that had been accepted up to that point; he noted its tendency to form acids by combining with many different substances, and he named the element oxygen (oxygène) from the Greek words for “acid former.”
- Lavoisier conducted the first accurate quantitative oxidation tests and explained combustion. In one experiment, Lavoisier found that heating tin and air in a closed container did not increase weight. As he opened the container, air rushed in, indicating that part of the stored air was used. He also noted that the tin had gained weight equivalent to the air that rushed back in. He showed that air contains vital air (needed for combustion and respiration) and azote. He has mistaken oxygen for a component of all acids and nicknamed necessary air oxygen. Chemists later realized Lavoisier was wrong, but the term had already stuck.
Occurrence of Oxygen
In the crust of the Earth, oxygen predominates. The crust, atmosphere, and hydrosphere make up Earth’s surface. Green plants absorb carbon dioxide in the presence of sunshine and create free oxygen, whereas animals and some microorganisms remove oxygen from the atmosphere and release it as carbon dioxide. About percent of the air’s free oxygen comes from photosynthesis.
- Oxygen makes up almost half of the earth’s crust’s mass (combined with other elements, principally silicon).
- Molecules of O2 and, to a lesser extent, O3 (ozone) may be found in the air we breathe.
- It accounts for around 20% of the air’s total mass. Combined oxygen accounts for around 89% of water’s bulk.
- Oxygen is a crucial component of living organisms, along with carbon, hydrogen, and nitrogen.
- It forms salt-like compounds, such as sulfates, carbonates, silicates, aluminates, and phosphates, by reacting with metals and nonmetals present in rocks to produce acidic (sulfur, carbon, aluminum, and phosphorus) or basic (calcium, magnesium, and iron) oxides.
- As oxygen in these solid complexes is tightly bound to metal atoms, removing it would be prohibitively costly, rendering the complexes unfeasible as oxygen sources despite their abundance.
- At room temperature, oxygen dissolves at a volumetric ratio of around 3 parts per 100 in freshwater and a bit less in seawater. Animals in the sea can only survive if there is enough oxygen in the water for them to breathe.
Isotopes of Oxygen
There are a total of 13 different isotopes of oxygen, ranging in mass from 12O to 24O. Natural abundances of the three oxygen isotopes 16O, 17O, and 18O are 99.8 percent, 0.04%, and 0.2 percent, respectively.
Oxygen Allotropes
Diatomic (O2) and triatomic (O3) oxygen are two allotropic forms of the element.
The diatomic form suggests that six electrons connect the atoms and two remain unpaired, explaining oxygen’s paramagnetism. Ozone molecules have an irregular shape because their three atoms are not in a line.
Ozone is a powerful oxidizing agent that breaks down many different types of chemicals into oxygenated derivatives such as aldehydes and acids, as well as converting sulfur dioxide to sulfur trioxide, sulfides to sulfates, iodides to iodine (providing an analytical technique for its measurement). Smog gets its foul smell and taste from the acids and aldehydes formed when ozone reacts with hydrocarbons in automobile exhaust. Commercial uses for ozone include those of a chemical reagent, disinfectant, sewage treatment, water purification, and bleaching agent for textiles.
Elemental Properties of Oxygen
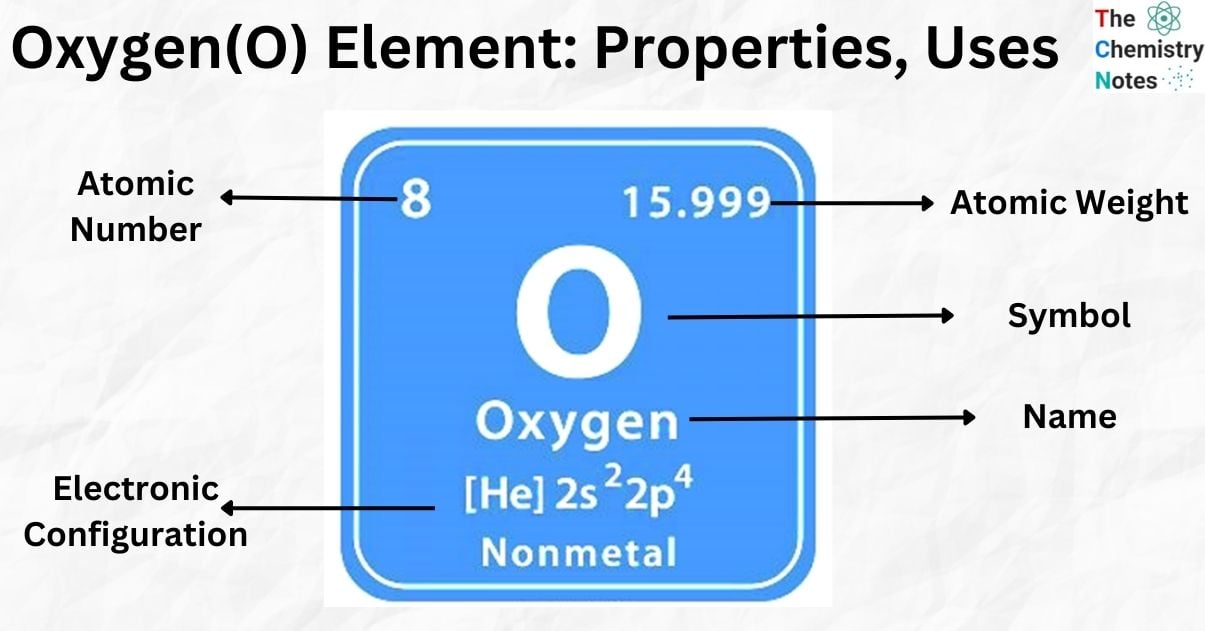
| Electronic Configuration | [He] 2s2 2p4 |
| Atomic Number | 8 |
| Atomic Weight | 15.999 g.mol -1 |
| State at 20°C | Gas |
| Group, Period, and Block | 16, 2, p-block |
| Density | 0.001308 g cm-1 |
| Covalent radius | 66 ± 2 pm |
| Van der Waals radius | 152 pm |
| Electron shells | 2, 6 |
Physical Properties of Oxygen
- Gaseous oxygen has no distinct smell, taste, or appearance.
- Both the liquid and the solid are light blue and very paramagnetic. The blue hue seen is the result of electromagnetic energy being absorbed from the red zone to the green visible region. Some solid oxygen forms have a reddish-black metallic appearance.
- Oxygen is necessary for combustion, can be combined with the vast majority of elements, and is found in hundreds of thousands of organic molecules.
- Highly reactive ozone (O3), is produced when an electrical discharge or UV light reacts with oxygen.
- Dioxygen produces weak charge-transfer complexes and is soluble in both water and organic solvents such as acetone and benzene.
- Oxygen dissolves at almost double the rate in water at 0 °C (14.6 mg/L) as it does at 20 °C (7.6 mg/L). At 25 degrees Celsius and 1 atmosphere (101.3 kPa), the oxygen content of freshwater is around 6.04 milliliters (mL) per liter, whereas the oxygen content of seawater is about 4.95 mL. Solubility in water is increased by 50% to 9.0 mL per liter at 5 °C and by 45% to 7.2 mL per liter in sea water.
- Oxygen freezes at 54.36 K (-218.79 °C, -361.82 °F), and condenses at 90.20 K (-182.95 °C, -297.31 °F).
| Melting Point | 54.36 K (-218.79 °C, -361.82 °F) |
| Boiling Point | 90.20 K (-182.95 °C, -297.31 °F) |
| Density | 0.001308 g cm-1 |
| Heat of Fusion | O2 0.444 kJ/mol |
| Heat of Vaporization | O2 6.82 kJ/mol |
| Molar Heat Capacity | O2 129.378 J/(mol·K) |
| Electronegativity | Pauling scale: 3.5 |
Chemical Properties of Oxygen
- At standard temperature and pressure (STP), two atoms of an element join together to form dioxygen, a colorless, odorless, tasteless diatomic gas with the formula O2.
- Oxygen is a nonmetallic element that is part of the chalcogen group on the periodic table. It is also a very reactive element. Because of this, it mixes easily (especially as oxides) with almost all other elements.
- Oxygen is a strong oxidizer, and after fluorine, it has the second-highest electronegativity of all reactive elements.
- By mass, oxygen is the third most common element in the universe, after hydrogen and helium. It is also the most common element in the Earth’s crust, making up almost half of its mass.
- Free oxygen is too chemically reactive to exist on Earth without the action of photosynthetic organisms, which use the energy from the sun to turn water into elemental oxygen. Elemental O2 didn’t start to build up in the atmosphere until about 2.5 billion years ago, when photosynthetic organisms started to evolve.
- Oxygen doesn’t burn, but fire needs it to keep going. This means that oxygen helps combustion happen.
- Oxygen can be a part of many different kinds of compounds. Some of the compounds are water, iron ore, carbon dioxide, etc.
- Reactivity: Ultraviolet (UV) light can react with oxygen in the stratosphere (a layer of the earth’s atmosphere) to make oxygen, and an electric discharge in oxygen can also make oxygen.
Uses and Applications of Oxygen
- It is the third most common element in the sun and the earth, and it is a part of the carbon-nitrogen cycle.
- Oxygen served as the foundation for all other elements’ atomic weight comparisons until 1961, when the International Union of Pure and Applied Chemistry decided to accept carbon 12 as the new baseline.
- The most significant application of the gas is found in the oxygen enrichment process that occurs in steel blast furnaces. For the production of ammonia, methanol, and ethylene oxide, a significant quantity is required of the synthesis gas. In addition, it is utilized in the bleaching of oils, the oxidation of organic compounds, the oxyacetylene welding process, and the analysis of the carbon content of steel and organic compounds.
- It finds widespread application in the metallurgical industry, particularly in the production of steel in blast furnaces and Bessemer converters.
- In many other chemical processes, it is also put to use in direct oxidation.
- In the field of chemistry, the odourless, odorless, and tasteless O2 gas molecule is utilized in the production of synthesis gas.
- It is put to use in the field of organic chemistry for the purpose of oxidizing organic hydrocarbons such as methane, ethane, ethylene, and acetylene, amongst others.
- It plays the role of an oxidizer for the fuels that are used in rocket propulsion.
- When there is an unexpected reduction in pressure within an aircraft, an emergency supply of oxygen will be made available to any passengers who are on board. This oxygen is not kept in the form of a gas but rather as the chemical sodium chlorate.
- Fish cannot survive without oxygen in the form of dissolved oxygen.
- As an explosive, a combination of liquid oxygen and powdered charcoal is often utilized.
Also Read: Carbon(C): Properties, Uses, Occurrence, History, Toxicity
Health and Environmental Effects of Oxygen
Health Effects
- In our bodies, oxygen is responsible for hundreds of different functions, the three most important of which are the creation of energy, the cleansing of the blood, and the elimination of toxins.
- A lack of oxygen can cause tiredness and weakness in the body, as well as a slowdown in the body’s natural detoxification processes, which can then result in a variety of illnesses, including metabolic disorders and cancer.
- The importance of ensuring that the proper amount of oxygen is present in the environment cannot be overstated in light of the increased levels of air pollution, modern lifestyles that are characterized by a lack of activity, and an increase in the intake of processed foods.
- Hemoglobin is absolutely necessary for the transportation of oxygen throughout our bodies. Myoglobin is responsible for the storage of oxygen in the muscular tissues where it is then released as required.
- The biosynthesis of many different molecules takes place in our body, and oxygen plays an important role in this process. Oxygen has the potential to turn certain lipid-soluble molecules into water-soluble ones, which can then be excreted.
- Oxygen is necessary for the existence of all kinds of life since it is a component of DNA as well as practically all other molecules that are significant to biology.
- To be able to breathe, every human being need oxygen, but as is the case in many situations, having too much oxygen can be harmful. Lung injury can develop if an individual is subjected to high concentrations of oxygen for an extended period. Lung injury can be caused by breathing in 50-100% oxygen at normal pressure for an extended length of time. Those who have jobs that expose them to high levels of pure oxygen on a regular or potentially regular basis should have their lung function evaluated before commencing employment and periodically thereafter. Because oxygen is often held at very low temperatures, one should be sure to wear the appropriate clothing to prevent the freezing of any of their body’s tissues.
- Oxygen that has been compressed and stored in cylinders is used in the medical field to treat persons who suffer from respiratory conditions such as asthma and COPD. Patients in emergency departments and intensive care units get oxygen from these cylinders.
- Moreover, mountain climbers, scuba divers, and astronauts all need it in order to perform their jobs. In addition, oxygen gas can be utilized to eliminate the germs that cause gangrene and can reverse the effects of carbon monoxide poisoning.
- Oxygen helps to create a protective ozone layer in the stratosphere, which sits between the planet and the sun. This layer absorbs potentially damaging ultraviolet (UV) radiation from the sun, preventing those rays from having a negative impact on the earth’s life forms and ecosystems.
Environmental Effects
- Sources of oxygen that have a high concentration and consequently encourage quick combustion provide a risk of fire and explosion when present in an environment that also contains fuels.
- Both the combustion of fossil fuels, which takes oxygen out of the atmosphere, and deforestation, which cuts down on oxygen generation, are contributing factors in today’s low levels of atmospheric oxygen.
- Since the pure oxygen environment was at normal atmospheric pressure rather than the one third pressure that would be utilized during a real launch, the fire that killed the Apollo 1 crew on a test launchpad spread so quickly. This was one of the contributing factors that contributed to their deaths.
Toxicity, Safety, and Precautions
- After just two to three hours of exposure to pure oxygen at two or more atmospheres, one may experience symptoms of central nervous system poisoning. These symptoms include dizziness, convulsions, and loss of consciousness.
- Smoking, which is linked with coughing and breathing difficulties, makes retrosternal discomfort worse, and exposure to cold air can cause it after inhaling pure oxygen at atmospheric pressure for several hours. Both of these conditions are connected with difficulty breathing.
Safety Measures
- In any circumstance when there has been an excessive intake of oxygen, immediate medical intervention is required. It is imperative that anyone working in rescue be aware of the significant fire threats that are linked with oxygen-rich environments. Those that are conscious should be led to an area that is not polluted so that they can breathe clean air.
- They should be kept in a calm and warm environment. The most essential thing is to evacuate the hazardous area as soon as possible. It is important that the attending physician is made aware that the patient has undergone hyperoxia.
- Wash your eyes thoroughly with a lot of water as soon as possible, being sure to intermittently raise both your upper and lower eyelids. Look for any contact lenses and take them out if you find any. Continue the rinsing process for at least ten minutes. Seek medical care.
- Water should be used liberally to flush infected skin. Take off any shoes and clothes that may have been affected. If symptoms develop, it is important to get medical assistance. Before reusing clothing, wash it first. Before being reused, shoes should have a thorough cleaning.
- Take the sufferer outside into the fresh air and keep them still in a posture that makes it easy for them to breathe. If the individual is not breathing, their breathing is erratic, or they have respiratory arrest, qualified people should administer artificial respiration or oxygen. It is possible that the person delivering help might be put in harm’s way by doing mouth-to-mouth resuscitation. Get medical treatment if the negative effects on your health continue or become severe. If the person is unconscious, they should be placed in the recovery position and emergency medical treatment should be sought. Keep your airway clear at all times. Loosen any clothing that is too snug, such a collar, a tie, a belt, or a waistline.
Precautions
- Exposure to airborne pollutants among workers can be reduced with adequate general ventilation.
- To guarantee compliance with environmental protection laws, it is important to inspect emissions from ventilation or work process equipment. To decrease emissions to safe levels, it may be required to install fume scrubbers, filters, or make other engineering adjustments to the process equipment.
- After working with chemicals, before eating, smoking, or using the restroom, and again when you’re done for the day, it’s important to wash your hands, arms, and face completely.
- It’s important to properly dispose of any clothing that may have been contaminated.
- Do laundry before wearing soiled items. Put eyewashes and safety showers in close proximity to the work area.
- When a risk assessment reveals that protecting one’s eyes from liquid splashes, mists, gases, or dusts is important, safety eyewear meeting an established standard should be used. Safety glasses with side shields should be worn if contact is feasible, unless the risk assessment calls for a higher level of protection.
- If a risk assessment shows that chemical-resistant, impermeable gloves that meet an established standard must be worn at all times when handling chemical items, then they must be worn at all times. Verify, in light of the specifications set out by the glove maker, that the gloves are still providing the necessary level of protection. It’s worth noting that various glove manufacturers may experience varied breakthrough times for the same glove material. The amount of time the gloves will protect a combination cannot be predicted if the components in the mixture are unknown.
- Selecting the appropriate body PPE for the job at hand and having it authorized by an expert is necessary before working with this product.
Before handling this product, you should see an expert to determine the best footwear and skin protection measures to use, based on the work at hand and the associated hazards. - Choose a respirator that has been certified to the appropriate standard based on the nature of the hazard and the likelihood of exposure. A respiratory protection program should be followed when using respirators to guarantee correct sizing, training, and other essentials. Known or predicted exposure levels, product dangers, and the respirators’ safe working limits should all factor into the final decision.
Handling and Storage
- Avoid dragging, rolling, sliding, or dropping cylinders to avoid any potential damage. Do not remove the valve cover from the cylinder until after you have moved it. Do not try to carry a cylinder by its cap, since that is designed to keep the valve from becoming damaged.
- Even if you’re just going a short distance, you should still utilize a cart (trolley, hand truck, etc.) made specifically for hauling cylinders. Never force anything (such as a screwdriver, hammer, or other tool) into a cap opening; doing so might damage the valve and lead to leaks.
- Remove corroded or overly-tightened caps with the use of an adjustable strap wrench. Turn the valve open slowly. The valve should be replaced and the manufacturer contacted if it is difficult to open. Keep the container’s valve closed at all times, even when it’s empty.
- Never expose any component of the container to direct flame or confined heat. Damage to the container and early failure of the pressure relief system, releasing the contents, are both possible outcomes in environments with high temperatures.
- Put away in a cool, dry place where the temperature won’t rise beyond 52 degrees Celsius (125 degrees Fahrenheit). Put up signs saying “No Smoking/No Open Fire” in all locations that will be used for either storage or usage. There can be no ignition sources.
- Use relevant standards and requirements (such as NFPA 30, NFPA 55, NFPA 70, and/or NFPA 221, in the United States) or the requirements specified by the Authority Having Jurisdiction to properly segregate packages and safeguard against potential fire and/or explosion damage (AHJ).
- Be sure to always keep containers upright so they can’t be accidentally knocked over. If a valve protection cap is included, make sure to screw it on securely before putting the container away. Keep containers in two categories, full and empty.
- Avoid wasting space by keeping containers that are already full for extended periods of time by using a FIFO inventory system. Refer to the product’s warning label for more safety information.
Information from Safety Data Sheet by Sigma Aldrich
References
- Cook, Gerhard A.; Lauer, Carol M. (1968). “Oxygen”. In Clifford A. Hampel (ed.). The Encyclopedia of the Chemical Elements. New York: Reinhold Book Corporation.
- Emsley, John (2001). “Oxygen”. Nature’s Building Blocks: An A–Z Guide to the Elements. Oxford, England: Oxford University.
- Raven, Peter H.; Evert, Ray F.; Eichhorn, Susan E. (2005). Biology of Plants (7th ed.). New York: W. H. Freeman and Company Publishers.
- Dole, Malcolm (1965). “The Natural History of Oxygen” (PDF). The Journal of General Physiology. 49 (1): 5–27. doi:10.1085/jgp.49.1.5
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 793. ISBN 0-08-037941-9.
- Priestley, Joseph (1775). “An Account of Further Discoveries in Air”. Philosophical Transactions. 65: 384–94.
- Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
