The element Nitrogen belongs to Periodic Table Group 15. It is a nonmetallic element that accounts for approximately 78 percent of the Earth’s atmosphere. It exists in the form of diatomic molecules, N2. The gas is inert due to a triple bond between the two nitrogen atoms.
It does not react with oxygen in the atmosphere under normal conditions, but it can be reacted in the laboratory to produce nitric and nitrous oxides. Nitrous oxide can be converted further to nitric acid.
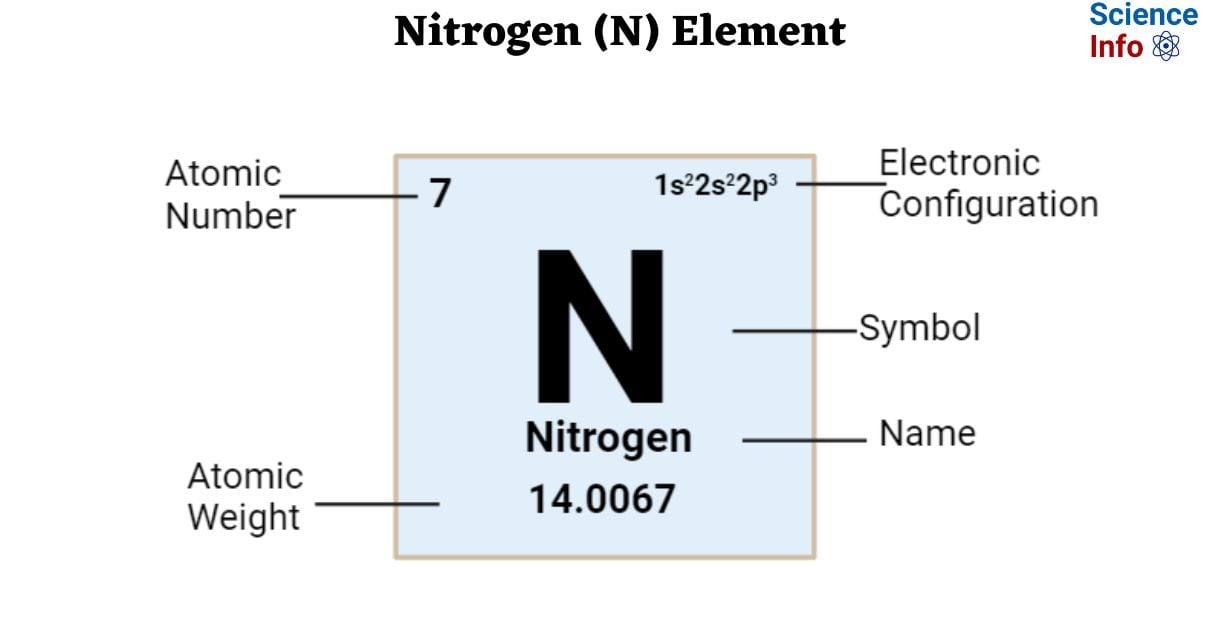
The Symbol of the element Nitrogen is N.
Interesting Science Videos
Nitrogen History
Nitrogen makes up roughly four-fifths of the Earth’s atmosphere, and it was isolated and identified as a distinct substance during early air research.
Scientists Contribution
Carl Wilhelm Scheele, a Swedish chemist, demonstrated in 1772 that air is a mixture of two gases, one of which he dubbed “fire air” because it aided combustion, and the other “foul air” because it remained after the “fire air” had been depleted
Nitrogen was recognized by a Scottish botanist named Daniel Rutherford (who was the first to publish his findings), a British chemist named Henry Cavendish, and a British clergyman and scientist named Joseph Priestley, who is credited with discovering nitrogen alongside Scheele.
French chemist Jean-Antoine-Claude Chaptal named Nitrogen in 1790.
Antoine-Laurent Lavoisier, whose explanation of the role of oxygen in combustion eventually overthrew the phlogiston theory, an incorrect view of combustion that became popular in the early 18th century, was the first to consider nitrogen a chemical element.
Lavoisier named it azote, because of nitrogen’s inability to support life which is still the French equivalent of nitrogen.
Occurrence of Nitrogen Element
Nitrogen is the sixth most abundant element in the universe. The Earth’s atmosphere is 75.51 percent by weight (or 78.09 percent by volume) nitrogen; this is the primary source for commerce and industry. There are also trace amounts of ammonia and ammonium salts in the atmosphere, as well as nitrogen oxides and nitric acid. This accounts for approximately 16% of the complex organic compounds known as proteins, which are found in all living organisms. It is naturally abundant in the Earth’s crust at 0.3 parts per 1,000.
Nitrogen in mineral deposits
Nitre or saltpetre (potassium nitrate, KNO3)
Chile saltpetre (sodium nitrate, NaNO3)
It is found in the rain and soil as ammonia and ammonium salts and in seawater as ammonium (NH4+), nitrite (NO2), and nitrate (NO3) ions in combination.
Isotopes of Nitrogen
It has two isotopes: N14 and N15.
N14 is 99.636 percent more abundant than N15.
Nitrogen Allotropes
An allotrope is one of the physical forms that an element can take. Unlike many other elements, it has only one natural allotrope: dinitrogen (N2).
Elemental Properties
| Electronic Configuration | 1s22s22p3 |
| Atomic Number | 7 |
| Atomic Weight | 14.0067 |
| Group, Period, and Block | 15, 2, p-block |
| Atomic Radius | 1.55 Angstrom |
| Covalent Radius | 0.71 Angstrom |
| Electronegativity | 3.04 (Pauling scale) |
Bonding in Nitrogen
A nitrogen atom’s electron configuration is 1s2 2s2 2p3.
It must gain three electrons to complete the outer shell of electrons. It has five electrons in its outermost shell and requires three more to achieve a stable configuration. As a result, two nitrogen atoms combine to form a molecule that shares three pairs of electrons.
Triple covalent bonds are formed when the atoms combine to form molecules with three pairs of shared electrons. The triple covalent bonding is extremely strong, with a bond energy of nearly 1000 kJ/mol.
Polarity of Nitrogen
Due to the strength of its triple bond and lack of polarity, it is very inert.
The electrons in its molecule are distributed evenly among the two nitrogen atoms.
As a result, nitrogen molecules are nonpolar, so it does not attract or interact with other molecules in the same way that polar molecules do.
Physical Properties of Nitrogen Element
- It has an atomic number of seven and is represented by the symbol ‘N.’
- At room temperature, it is a diatomic molecule.
- Colorless and odorless gas.
- Fifth most abundant element on the planet.
- Nonmetal.
- It is not poisonous, but animals die in nitrogen atmospheres due to a lack of oxygen.
- It has a very low water solubility (23.2 cm3 per liter of water at standard pressure)
| Melting Point | −209.86 °C (−345.8 °F) |
| Boiling Point | −195.8 °C (−320.4 °F) |
| Density | 0,001145 g/cm3 |
Chemical Properties of Nitrogen Element
- At room temperature, N2 is almost non-reactive. The chemical inertness of N2 at room temperature is due to the molecule’s high stability.
- It does not burn or support combustion.
- The two nitrogen atoms in a N2 molecule are linked together by a triple bond. The bond enthalpy of the triple bond is extremely high (the amount of heat energy required to break a chemical bond).
- The first Ionization energy of N2 is 1402 KJ/mol.
- N2 is almost unreactive to most reagents due to its extremely high bond dissociation enthalpy.
- At high temperatures, however, it combines with some metals and nonmetals to form ionic and covalent compounds known as nitrides.
Uses and Applications of Nitrogen Element
- Used in the production of ammonia, the production of nitric acid, and subsequent use as a fertilizer.
- Important compounds found in nitric acid salts include potassium nitrate, ammonium nitrate, and nitric acid. Nitro glycerine and other nitrated organic compounds are frequently used as explosives.
- Liquid nitrogen is used as a refrigerant in food transportation and freezing. It is also used in the preservation of bodies and reproductive cells, as well as the stable storage of biological samples. It is also used in the food industry to prevent spoilage caused by oxidation, mold, or insects.
- It constitutes 78 percent of the Earth’s atmosphere and is found in all living tissue. Also, it is an essential component of life because it is a component of DNA and thus a part of the genetic code.
- Used in the chemical industry to prevent oxidation or other deterioration of a product, as an inert diluent of a reactive gas, as a carrier to remove heat or chemicals, and as a fire or explosion inhibitor.
- Used in the electrical industry to prevent oxidation and other chemical reactions and to pressurize cable jackets and shield motors.
- In the metals industry, it helps prevent oxidation, carburization, and decarburization during welding, soldering, and brazing.
- As a nonreactive gas, it is used to create foamed (or expanded) rubber, plastics, and other materials. as a propellant gas for aerosol cans, and to pressurize reaction jet liquid propellants.
- Liquid nitrogen has also been found to be useful in cryogenic research.
- It promotes the development of vegetative bodies. It raises the quality of green leafy vegetables and fodder crops. An important component of chlorophyll is proteins, enzymes, and amino acids. It stimulates the growth of root uptake and the development of cations.
Health and Environmental Effects of Nitrogen
- When excess nitrogen from the atmosphere returns to the earth, it can harm the health of forests, soils, and waterways.
- Suffocation by a chemical (gas or vapor) can result in death or unconsciousness.
- formation of pollutants like ammonia and ozone can impair our ability to breathe, reduce visibility, and alter plant growth.
- Algae grow faster than ecosystems can handle. Significant increases in algae harm water quality, food resources, and habitats and reduce the oxygen required by fish and other aquatic life.
- Deterioration of soil microbes and acidification.
- Rising global temperatures lead to photochemical smog.
- Ground-water pollution.
Toxicity, Safety, and Precaution
Toxicity: Nitrogen is not toxic because it makes up approximately 78 percent of the air we breathe. Nitrogen gassing differs from hydrogen sulfide in its mechanism (H2S). While H2S has a well-documented direct toxic effect, nitrogen-rich atmospheres will cause asphyxiation due to a decrease in the oxygen content of the inhaled gases. The physiological effects of varying degrees of oxygen deficiency are well documented as well.
| Oxygen (%vol) | Effects and Symptoms |
| 15-19 | The first sign of hypoxia. Decreased ability to work strenuously. |
| 12-14 | Respiration increases with exertion, pulse up, impaired muscular coordination, perception, and judgment |
| 10-12 | Respiration further increases in rate and depth, poor judgment, lips blue |
| 8-10 | Mental failure, fainting, unconsciousness |
| 6-8 | 6 minutes – 50% probability of death, 8 minutes – 100% probability of death |
| 4-6 | Coma in 40 seconds, convulsions, respiration ceases, death |
What is an Asphyxiant?
Suffocation by a chemical (gas or vapor) can result in death or unconsciousness.
Nitrogen and other simple asphyxiants displace oxygen in the atmosphere.
They are particularly dangerous in confined or enclosed spaces.
Chemical asphyxiants, such as carbon monoxide and hydrogen sulfide, impair the body’s ability to absorb and transport oxygen to tissues.
Safety Precautions
- Different safety regulations are available for guiding the use of nitrogen for industrial purposes. Hence, the first and foremost thing is to read the safety guidelines.
- Regardless of industrial location, all personnel must be trained on the proper use of personal protective devices as well as proper actions to carry out in case of accidental hazardous exposure.
- Continuous atmospheric monitoring is the most important. Additionally, the ability to sample atmospheric air within these spaces will aid in determining its suitability for breathing. The following tests are essential: Concentrations of oxygen, presence of combustible gas, and toxic vapor testing.
- Adequately Ventilated Spaces: Induced draft air circulation in restricted areas will substantially reduce the buildup of noxious gases as well as reduce the likelihood of fatal exposure.
- Overall the best way to prevent asphyxiation is to properly train all personnel involved in industrial manufacturing processes.
Cost or Pricing of Nitrogen Element
| NITROGEN <2 PPM MOISTURE | 99.995 | 255 | $12.93 |
| NITROGEN <2 PPM MOISTURE | 99.995 | 304 | $15.28 |
| NITROGEN (PP) | 99.998 | 257 | $46.51 |
| NITROGEN (UHP) | 99.999 | 257 | $54.26 |
| NITROGEN (ZERO) | 99.998 | 200 | $64.52 |
| NITROGEN (6000 PSIG-HIGH PRESSURE) | 99.998 | 500 | $147.66 |
References
- https://www.britannica.com/science/nitrogen#ref280541
- https://www.lenntech.com/periodic/elements/n.htm
- https://alevelchemistry.co.uk/notes/nitrogen-and-sulphur/
- https://unacademy.com/content/nda/study-material/chemistry/nitrogen-physical-and-chemical-properties-of-nitrogen/
- https://www.chemicool.com/elements/nitrogen.html
- https://pmt.physicsandmathstutor.com/download/Chemistry/A-level/Notes/CIE/AS-Inorganic-Chemistry/Detailed/13.%20Nitrogen%20and%20Sulfur.pdf
- Reactive nitrogen and human health: acute and long-term implications 9https://pubmed.ncbi.nlm.nih.gov/12078000/0
