The main component of the nitrogen cycle is the element nitrogen in the air. The air we breathe is composed of 78% nitrogen, 21% oxygen, and other trace gases. Nitrogen (N) is a necessary component of the building blocks of life, DNA, RNA, and proteins. To live and grow, all organisms require nitrogen. Although N2 makes up the majority of the air we breathe, most of the nitrogen in the atmosphere is unavailable to the use of organisms. This is due to the strong triple bond formed by the N atoms in N2 molecules, which forms it relatively inert, or unreactive, whereas organisms require reactive nitrogen to incorporate it into cells.
Nitrogen must first be transformed from N2 gas into a more chemically usable form, such as ammonium (NH4+), nitrate (NO3–), or organic nitrogen (for example, urea (NH2)2CO). The nitrogen cycle is composed of the changes that nitrogen goes through as it travels between the atmosphere, the land, and living beings. The nitrogen cycle is crucial to ecology. It involves a number of chemical reactions, including the fixation of nitrogen, nitrification, denitrification, decay, and putrefaction.
Interesting Science Videos
Importance of Nitrogen
- Nitrogen is a necessary component of nucleic acid DNA. DNA is found in nearly all living organisms as the primary component of chromosomes, which contain genetic information.
- Plants cannot produce amino acids if they do not receive enough nitrogen (substances that contain nitrogen and hydrogen and make up many living cells, muscles, and tissue).
- Plants cannot produce the special proteins required by plant cells if amino acids are not present.
- Plant growth suffers when there is insufficient nitrogen.
The delicate balance of substances necessary for life is an important area of study, and hence nitrogen balance is an important topic of discussion.
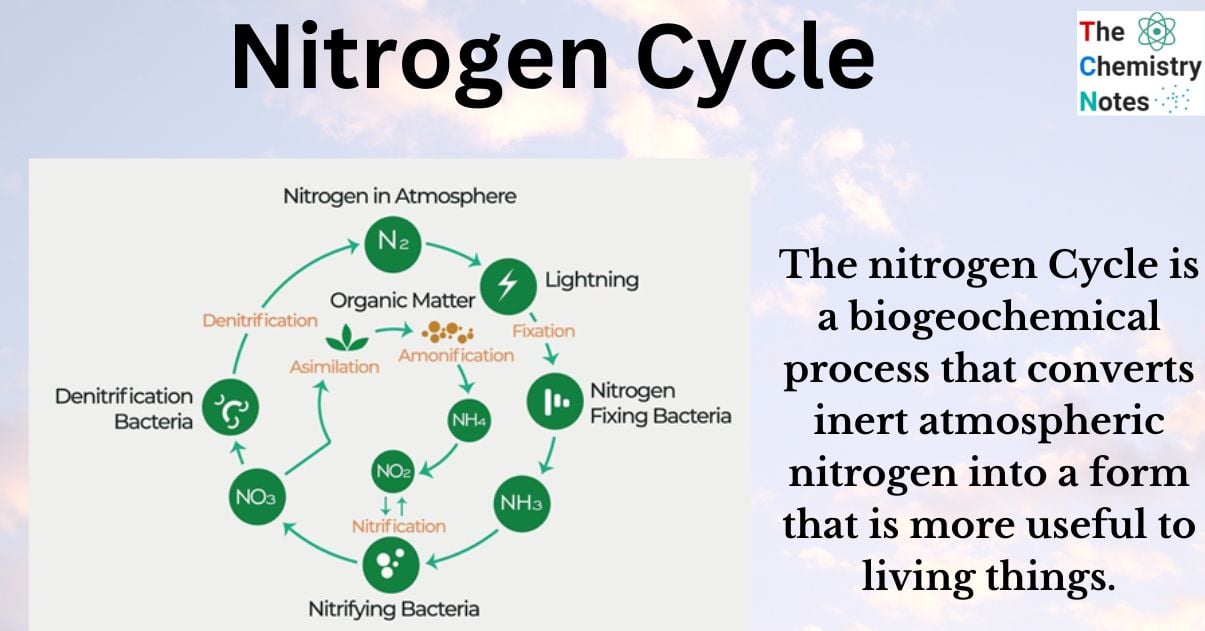
Nitrogen Cycle
The nitrogen Cycle is a biogeochemical process that converts inert atmospheric nitrogen into a form that is more useful to living things.
The Nitrogen Cycle discusses about the conversion of nitrogen into various forms before passing from the atmosphere to the soil to organisms and back into the atmosphere. Therefore, the nitrogen cycle describes the movement of nitrogen in the environment and has numerous significant effects on the environment.
Steps in the Nitrogen Cycle
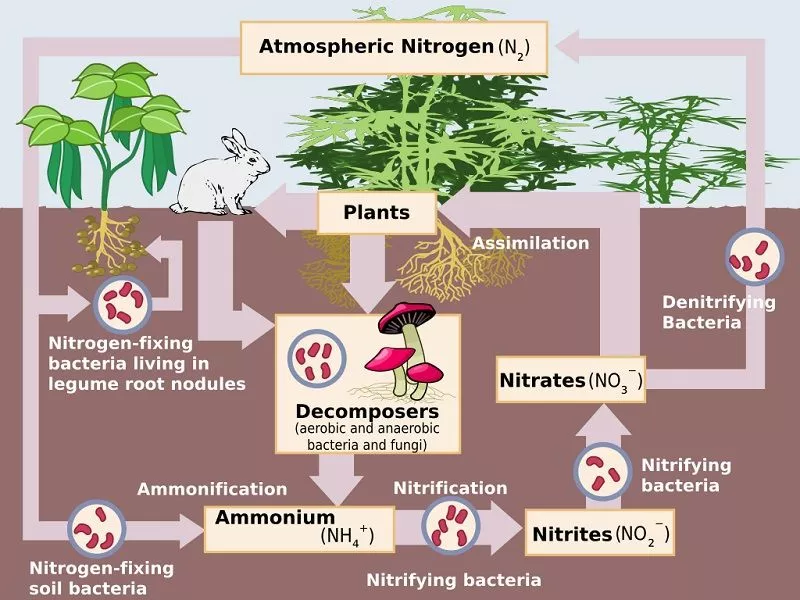
The nitrogen cycle consists of four major steps, which are as follows:
- Nitrogen Fixation
- Ammonification/ Decay
- Nitrification
- Assimilation
- De-nitrification
Nitrogen Fixation
The nitrogen cycle starts with this step. This step is distinguished by the transformation of atmospheric N2 into ammonia (NH3). Plants can use a nitrogen-based molecule called ammonia (NH3).
N2 + H+ → NH3 + H2
Bacteria such as Azotobacter and Rhizobium play an important role in this process. They live in the roots of leguminous plants and aid in the conversion of inert nitrogen to ammonia.
Nitrogen fixation can take place in one of three ways:
- Fixation of the atmosphere (via lightning),
- industrial fixation (manufacturing ammonia under high temperature and pressure conditions)
- Biological nitrogen fixation (Bacteria such as Rhizobium and blue-green algae)
Ammonification
The soil contains the buried remains of dead plants and animals. They decompose and produce ammonia, carbon dioxide, and water with the assistance of fungi such as actinomyces. Ammonification is the process by which ammonia is formed. Ammonia already exists in the soil due to nitrogen-fixing bacteria. Ammonification raises the ammonia concentration in the soil.
Nitrification
The presence of bacteria in the soil converts ammonia into nitrate during this process. Nitrites are formed through the oxidation of ammonia by Nitrosomonas bacteria species. Nitrobacter then converts the nitrites produced into nitrates. This conversion is critical because ammonia gas is toxic to plants.
The following is the reaction involved in the Nitrification process:
Conversion of Ammonia into Nitrites: 2NH3 + 3O2 → 2NO2– + 2H+ + 2H2O
Conversion of Nitrites to Nitrates: 2NO2– + O2 → 2NO3–
Assimilation
The absorption of nitrates and other nitrogen compounds is known as assimilation. Nitrogen compounds are required for the formation of critical biomolecules. Plants use their roots to absorb nitrogen compounds from the soil, which are available in the form of ammonia, nitrite ions, nitrate ions, or ammonium ions and are used in the formation of plant and animal proteins.
Denitrification
Since nitrogen is a gas and is quickly lost to the atmosphere once it is converted to dinitrogen, it is unlikely that it will ever be converted back into a form that is biologically accessible. The nitrogen transformation that removes nitrogen from ecosystems is denitrification.
NO3– → N2+ N2O
Denitrification is an anaerobic process in which denitrifying bacteria convert nitrate to dinitrogen in the following order:
NO3– → NO2– → NO → N2O → N2
Some nitrates are not absorbed by plants. Pseudomonas and Clostridium help to convert them into atmospheric nitrogen. This is the final step in which the nitrogen compounds present in the soil are returned to the atmosphere.
Importance of Nitrogen Cycle
The nitrogen cycle is significant for the following reasons:
- The nitrogen cycle is important because it is necessary for plants to produce chlorophyll, which makes it essential to their existence.
- Animals receive nitrogen and nitrogen molecules from plants because plants require nitrogen in their metabolic activities. Nitrogen is necessary since it is an essential component of cell composition.
- The bacteria support the ammonification process by assisting in the breakdown of decaying animal and plant debris. Nitrates and nitrites are released into the soil as a consequence of the nitrogen cycle, which serves to replenish the soil with nutrients needed for agriculture, and thus adds to the natural cleansing of the environment.
Nitrogen Reservoirs and Forms
The atmosphere’s (∼4 × 109 Tg) of dinitrogen (N2) gas is the planet’s greatest surface-based source of nitrogen. Additionally, the crust and ocean sediments contain a significant geological reservoir of nitrogen that is not N2, such as organic nitrogen found in sediments and sedimentary rocks and ammonium found in silicate minerals and clays.
| Nitrogen form | Molecular formula | Redox state |
| Ammonium, ammonia | NH4 +, NH3 | −3 |
| Organic N | R-NH3 | -3 |
| Hydrazine | N2H4 | -2 |
| Hydroxylamine | NH2OH | -1 |
| Dinitrogen | N2 | 0 |
| Nitrous acid | NO2−, HNO2 | +3 |
| Nitrate, nitric acid | NO3 −, HNO3 | +5 |
Global Nitrogen Cycle and Human-Induced Change
The worldwide cycling of nitrogen (N) in various chemical forms between the atmosphere, land, and ocean reservoirs is the outcome of the biotic and abiotic transformations of N as well as its physical conveyance by rivers and the atmosphere. Since the Industrial Revolution, human activities such as food production and the burning of fossil fuels have disrupted the global N cycle by adding additional N to terrestrial and marine permanent N.
The Haber Bosch method for synthetic N2 fixation to ammonium was developed in the early 20th century, and it allowed for the industrial-scale manufacturing and widespread use of N-rich fertilizers.
Due to this, agricultural yields that were previously constrained by the quantity of naturally occurring fixed N have been able to increase to levels that can feed fast-expanding populations. Other significant human-derived N sources include the burning of fossil fuels, which releases nitrogen oxides (NOx), and biological N2 fixation related to agriculture (such as the cultivation of legumes). Human-induced N has advantages for human society, but there are also huge costs to the environment and to human health.
Eutrophication
Eutrophication is the process through which a body of water becomes too nutrient-rich, resulting in the abundant development of simple plant life. An indication of this process is an excessive proliferation (or bloom) of plankton and algae in a body of water. Since eutrophication frequently leads to the degradation of water quality and the reduction of dissolved oxygen in water bodies, it is seen as a severe environmental problem. Eutrophic waters have the potential to eventually turn into “dead zones” that cannot sustain life.
Causes of Eutrophication: The expansion of plant life in an ecosystem is constrained by the availability of nutrients like nitrogen and phosphorus. Algae, plankton, and other basic plant life grow more readily in water bodies that are too supplied with these nutrients than do more sophisticated plant life.
References
- Risgaard-Petersen, N. et al. Evidence for complete denitrification in a benthic foraminifer. Nature 443, 93–96 (2006).
- Strous, M. et al. Missing lithotroph identified as new planctomycete. Nature 400, 446–449 (1999).
- Howarth, R. W. Coastal nitrogen pollution: a review of sources and trends globally and regionally. Harmful Algae 8, 14–20. (2008).
- Johnson, P. T. J. et al. Linking environmental nutrient enrichment and disease emergence in humans and wildlife. Ecological Applications 20, 16–29 (2010).
- https://www.earth.com/earthpedia-articles/why-is-the-nitrogen-cycle-so-important/
- Kim, Haryun; Lee, Kitack; Lim, Dhong-Il; Nam, Seung-Il; Kim, Tae-Wook; Yang, Jin-Yu T.; Ko, Young Ho; Shin, Kyung-Hoon; Lee, Eunil (2017-05-11). “Widespread Anthropogenic Nitrogen in Northwestern Pacific Ocean Sediment”. Environmental Science & Technology. 51 (11): 6044–52. Bibcode:2017EnST…51.6044K. doi:10.1021/acs.est.6b05316. ISSN 0013-936X. PMID 28462990.
- Global Nitrogen Cycle: Critical Enzymes, Organisms, and Processes for Nitrogen Budgets and Dynamics. Xinning Zhang* Bess B. Ward Daniel M. Sigman https://doi.org/10.1021/acs.chemrev.9b00613
