Chemical equations can be written in a variety of ways. Unbalanced equations, which indicate the species involved; balanced chemical equations, which indicate the number and type of species; molecular equations, which express compounds as molecules rather than component ions; and net ionic equations, which only deal with the species that contribute to a reaction, are among the most common.
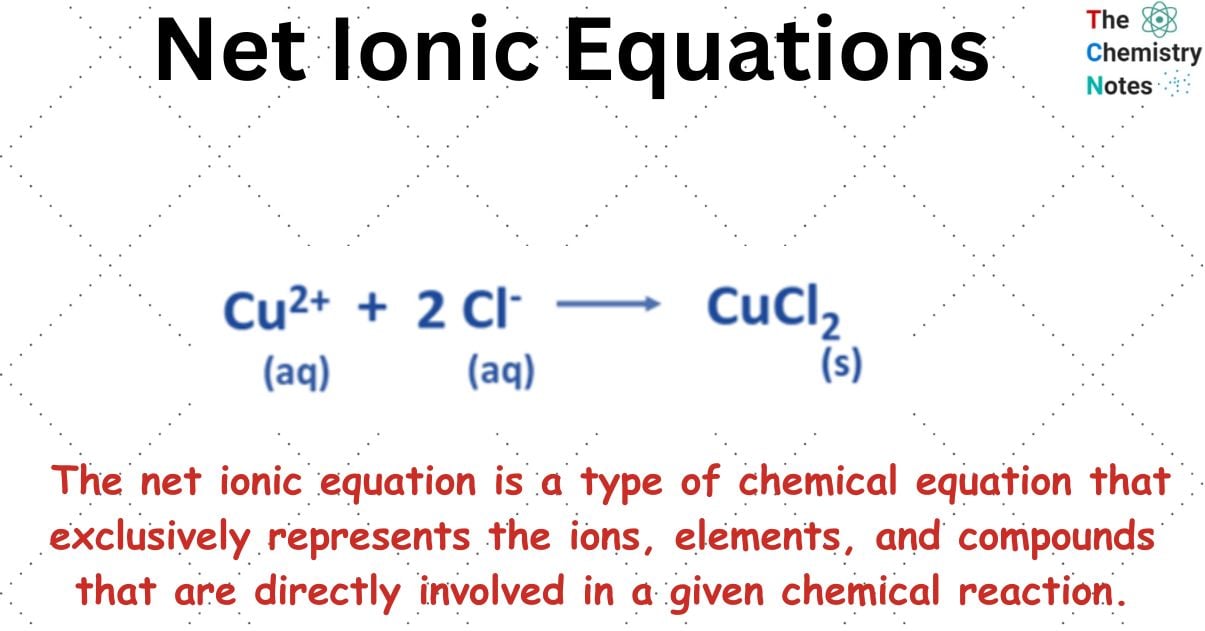
What are Net Ionic Equations?
The net ionic equation is a type of chemical equation that is utilized specifically for solutions that are aqueous in nature. The net ionic equation is a type of chemical equation that exclusively represents the ions, elements, and compounds that are directly involved in a given chemical reaction.
The diagram exclusively depicts the ions and molecules that participate in the chemical reaction. All the species participating in the chemical reaction are in the ionic form. Frequently, a precipitate is formed by one or more of the products. It is possible to ascertain the solubility or insolubility of a substance by utilizing the solubility rules.
The net ionic equation finds frequent application in acid-base neutralization reactions, double displacement reactions, and redox reactions. Stated differently, the net ionic equation pertains to chemical reactions that exhibit strong electrolytic properties when dissolved in aqueous solutions.
Steps to Write Ionic Equations
Step 1: It is important to note that there exists a distinction between ionic and molecular substances. The initial stage in constructing a net ionic equation is to identify the ionic compounds that participate in the reaction. Ionic compounds are characterized by possessing a charge that enables them to undergo ionization when dissolved in an aqueous medium. Compounds that lack an electrical charge are commonly referred to as molecules. These compounds are occasionally referred to as covalent compounds due to their formation between two non-metallic elements.
Step 2: Determine the solubility of a given compound. Not all ionic compounds undergo dissociation into separate ions due to their limited solubility in an aqueous medium. Prior to proceeding with the remaining equation, it is imperative to ascertain the solubility of each individual molecule.
Step 3: Conduct an analysis of a chemical compound in order to identify its positively charged cation and negatively charged anion. Typically, the metallic elements constitute the positive ions, also known as cations, in a given compound. The anions are the negative non-metallic ions of the compound. Cations are invariably generated by metals, albeit certain non-metals possess the ability to generate them as well.
Step 4: Identify the polyatomic ions involved in the reaction. Polyatomic ions are electrically charged molecules that exhibit strong intermolecular forces, preventing their dissociation during chemical reactions. It is imperative to differentiate polyatomic ions due to their unique charge and inability to decompose into individual constituents. Polyatomic ions can exhibit both positive and negative charges.
Step 5: Achieve stabilization of the complete molecular equation. It is imperative to ensure complete balance of the initial equation prior to drafting a net ionic equation. The process of balancing a chemical equation involves the addition of coefficients to the compounds in order to achieve an equal number of atoms for each element on both sides of the equation.
Step 6: Ascertain the physical states of the constituent compounds. Typically, one may encounter nomenclature within a given problem that can elucidate the physical state of individual molecules. Several guidelines can be employed to ascertain the state of an element or compound.
Step 7: Determine the solubility of the species in the given solution, leading to their dissociation into positively charged cations and negatively charged anions. When dissociation occurs, a species or compound undergoes a separation into its positively charged (cation) and negatively charged (anion) constituents. These are the constituents that will ultimately be equilibrated in the net ionic equation.
Step 8: Ascertain the charge of each ion that has undergone dissociation. It is important to note that non-metals are typically classified as negative anions, while metals are categorized as positive cations. The utilization of the group number in the periodic table enables the determination of the corresponding charge of each element. It is imperative to ensure that the charges of all ions present in the molecule are equilibrated.
Step 9: Rewrite the equation using the constituent ions of soluble ionic compounds. Strong acids and other compounds that are capable of dissociation or ionization will separate into two distinct ions. The state of matter will remain (aq) even though the equation must remain balanced.
Step 10: Eliminating identical ions on both sides of the equation eliminates the spectator ions. Only if they are identical on both sides can we cancel the order. Delete all species that have been canceled from the action.
Net Ionic Equation with Examples
Write the complete ionic equation from the chemical reaction equation.
- An instance of a chemical reaction occurs when copper (II) sulfate and sodium chloride react in their aqueous states.
- Copper (II) sulfate is represented by the chemical formula CuSO4, whereas sodium chloride is denoted by the chemical formula NaCl.
- The balanced chemical equation presented below depicts the chemical reaction that occurs between CuSO4 and NaCl.
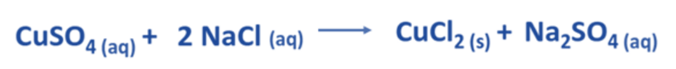
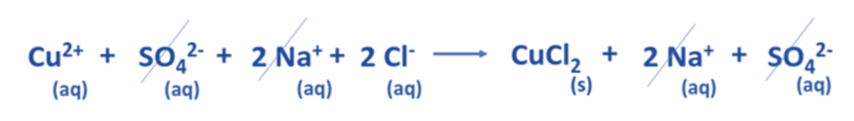
- In an aqueous solution, the dissociation of CuSO4 yields Cu2+ and SO42- ions on the reactant side. Likewise, in an aqueous solution, the dissociation of 2 NaCl results in the formation of 2 Na+ and 2 Cl– ions.
- Regarding the products, Na2SO4 undergoes dissociation into two Na+ and SO42- ions, whereas CuCl2 exists in a precipitated state and does not undergo dissociation into its constituent ions.
- The aforementioned chemical equation may be expressed as a comprehensive ionic equation, as demonstrated below.
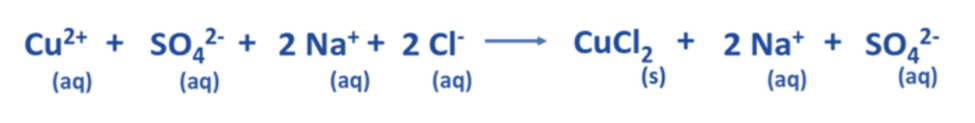
Determine the spectator ions present in the complete ionic equation.
- The aforementioned equation demonstrates that two Na+ ions and one SO42- ion remain constant on both the reactant and product sides.
- Spectator ions are those that remain unreactive throughout a chemical reaction.
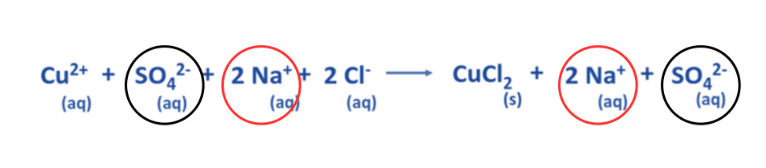
Eliminate the spectator ions present on either side of the chemical equation.
Verify that the net ionic equation is a balanced equation.
Derive the net ionic equation.
Now, the balanced net ionic equation for this reaction is:
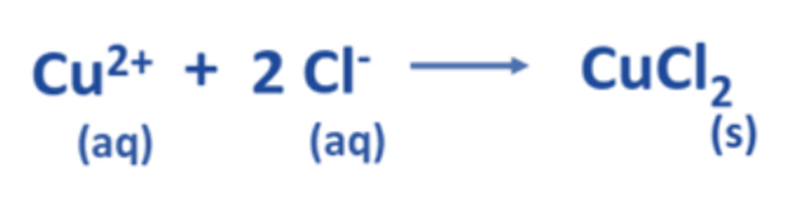
What are Spectator Ions?
Spectator ions refer to ions that are present in a chemical reaction but do not participate in the actual chemical change.
They remain in their original state and do not undergo any chemical reaction or change in oxidation state.
Important Tips for Writing the Net Ionic Equation
The ability to differentiate between molecular and ionic compounds, identify strong acids and bases, and make predictions regarding compound solubility is crucial in determining the dissociation of species into ions or formation of solids (precipitates). Molecular compounds such as sucrose or sugar exhibit a non-dissociative behavior when exposed to water. Ionic compounds, such as sodium chloride, undergo dissociation in accordance with established solubility principles. Strong acids and bases exhibit complete ionization, whereas weak acids and bases demonstrate only partial ionization.
- When dealing with ionic compounds, it is advisable to refer to the solubility rules. It is imperative to adhere to the established guidelines in a sequential manner.
- Solubility is a characteristic feature of alkali metal salts. For instance, the salts of Li, Na, K, and other elements can be referred to. In case of uncertainty, a periodic table may be consulted.
- All ammonium salts (NH4+) exhibit solubility.
- The solubility of salts containing NO3–, C2H3O2–, ClO3–, and ClO4 anions is high.
- The salts of Ag+, Pb2+, and Hg22+ exhibit insolubility.
- Salts containing Cl–, Br–, and I– ions exhibit solubility.
- The salts of CO32-, O2-, S2-, OH–, PO43-, CrO42-, Cr2O72-, and SO32- exhibit insolubility. Although there might be some exceptions.
- With some exceptions, all salts containing SO42-are soluble.
By adhering to these guidelines, one can determine that sodium sulfate exhibits solubility, whereas iron sulfate does not. - There exist six strong acids that undergo complete dissociation, namely HCl, HBr, HI, HNO3, H2SO4, HClO4.
- The oxides and hydroxides of alkali metals (group 1A) and alkaline earth metals (group 2A) are potent bases that undergo complete dissociation.
Examples of Net Ionic Equation
- Determine the net Ionic equation of a given chemical equation.
Mg(NO3)2 + Na2CO3 → MgCO3 + 2 NaNO3
Ionic Equation: Mg2+ + 2 NO3¯ + 2 Na++ CO32- → MgCO3 + 2 Na+ + 2 NO3¯
Net ionic equation: Mg2+ + CO32- → MgCO3
- Determine the net Ionic equation of a given chemical equation.
SrBr2 + K2SO4 → SrSO4 + 2 KBr
Ionic Equation: Sr2+ + 2 Br¯ + 2 K+ + SO42- → SrSO4 + 2 K+ + 2 Br¯
Net ionic equation: Sr2+ + SO42- → SrSO4
- Determine the net Ionic equation of a given chemical equation.
MnCl2 + (NH4)2CO3 → MnCO3 + 2 NH4Cl
Ionic Equation: Mn2+ + 2 Cl¯ + 2 NH++ CO32- → MnCO3 + 2 NH4+ + 2 Cl¯
Net ionic equation: Mn2+ + CO32- → MnCO3
References
- Helmenstine, Anne Marie, Ph.D. (2023, April 5). Net Ionic Equation Definition. Retrieved from https://www.thoughtco.com/net-ionic-equation-in-chemistry-604575
- https://www.studysmarter.us/explanations/chemistry/chemical-reactions/net-ionic-equations/
- https://www.geeksforgeeks.org/net-ionic-formula/
- https://www.expii.com/t/net-ionic-equation-overview-examples-8577
- https://topblogtenz.com/how-to-write-net-ionic-equations/
