The molecular orbital theory was developed by Hund and Mulliken in 1932 and later by Lennerd Jones and Coulson. This theory is also known as the Hund-Mulliken theory. In 1996, Mulliken was awarded the Nobel Prize in Chemistry. This theory is predicated on the idea that the electron state in a molecule may also be characterized as a group of molecular electron orbitals, each of which has a distinct set of molecular quantum numbers that correspond to them.MOT is currently the most precise description of molecular bonding.
According to the molecular orbital theory, all of the atomic orbitals of the atoms involved in the formation of a molecule are entirely mixed up and produce a set of new orbitals known as Molecular orbitals. These molecular orbitals now belong to the entire molecule and do not correspond to any of the bonding atoms. Therefore, MOT assumes that covalent molecules only have molecular orbitals and do not have atomic orbitals.
Electrons in molecules, like electrons in solitary atoms, are limited to discrete (quantized) energies. The term “molecular orbital” refers to the area of space where valence electrons in a molecule are most likely to be found. Like an atomic orbital, a molecular orbital is filled when it includes two electrons with opposite spin.
Bonding molecular orbitals
The in-phase combination of atomic orbitals results in a lower energy molecular orbital, in which most of the density of electrons is located directly between the nuclei. Electrons in these orbitals are attracted by both nuclei at the same time, making them more stable (lower in energy) than in isolated atoms. Adding electrons to these orbitals provides a force that holds the two nuclei together, therefore, they are known as bonding molecular orbitals.
Anti-bonding molecular orbitals
A higher energy molecular orbital with a node between the nuclei is produced by the out-of-phase addition, which can also be thought of as subtracting the wave functions. The asterisk indicates that the orbital is antibonding. Electrons in these orbitals are far from the region between the two nuclei. The attractive interaction between the nuclei and these electrons pulls these two nuclei apart. These orbitals are hence known as antibonding orbitals.
In the same way, as electrons fill lower energy atomic orbitals before higher energy atomic orbitals, they fill the lower energy bonding orbital before the higher energy antibonding orbital.
Comparison between Molecular Orbitals (MOs) and Atomic Orbitals (AOs)
- Electrons, whether in atomic or molecular orbitals, can be denoted by a specific wave function.
- The shapes and energies of molecular orbitals differ just like those of atomic orbitals.
- When entering AOS or MOS, electrons should follow the Aufbau principle, Pauli’s exclusion principle, and Hund’s rule.
- Like atomic orbitals, molecular orbitals can accommodate two electrons with opposite spins.
Formation of molecular orbitals
Molecular orbitals are generated by combining atomic orbitals of atoms. By using wave mechanics, atomic orbitals can be described in terms of wave function Ψ.
Although we can write the Schrodinger wave equation for an entire molecule, this is a challenging process. To solve this problem, scientists have developed the approximation method known as the Linear combination of atomic orbital (LCAO) technique.
Linear combination of atomic orbital (LCAO) approximation
The mathematical method of combining atomic orbitals to form molecular orbitals is known as a linear combination of atomic orbitals (LCAO). An electron’s wavelike features are described by the wave function. Atomic orbital wave functions combine to form molecular orbitals. Combining waves can result in constructive interference, where peaks line up with peaks, or destructive interference, where peaks line up with troughs. The three-dimensional waves in orbitals combine to create nodes, or areas with no electron density, and in-phase waves, which produce regions with a greater probability of electron density. LCAO is a convenient approach to explain how molecular orbitals are formed from atomic orbitals. Linear atomic orbitals wave functions result from simultaneous addition and subtraction overlap to generate two i.e., bonding and antibonding molecular orbitals.
Let us consider two A and B combined to form molecule AB. If Ψ A and Ψ B are the wave function of atomic orbitals A and B respectively and Ψ MO is the wave function of molecular orbitals of AB. The formation of molecular orbitals can be represented as:
Bonding molecular orbitals: Ψ A+ Ψ B → Ψ MO or ( Ψ AB)
Anti-bonding molecular orbitals: Ψ A– Ψ B → Ψ MO or ( Ψ AB)
Overlapping of different atomic orbitals
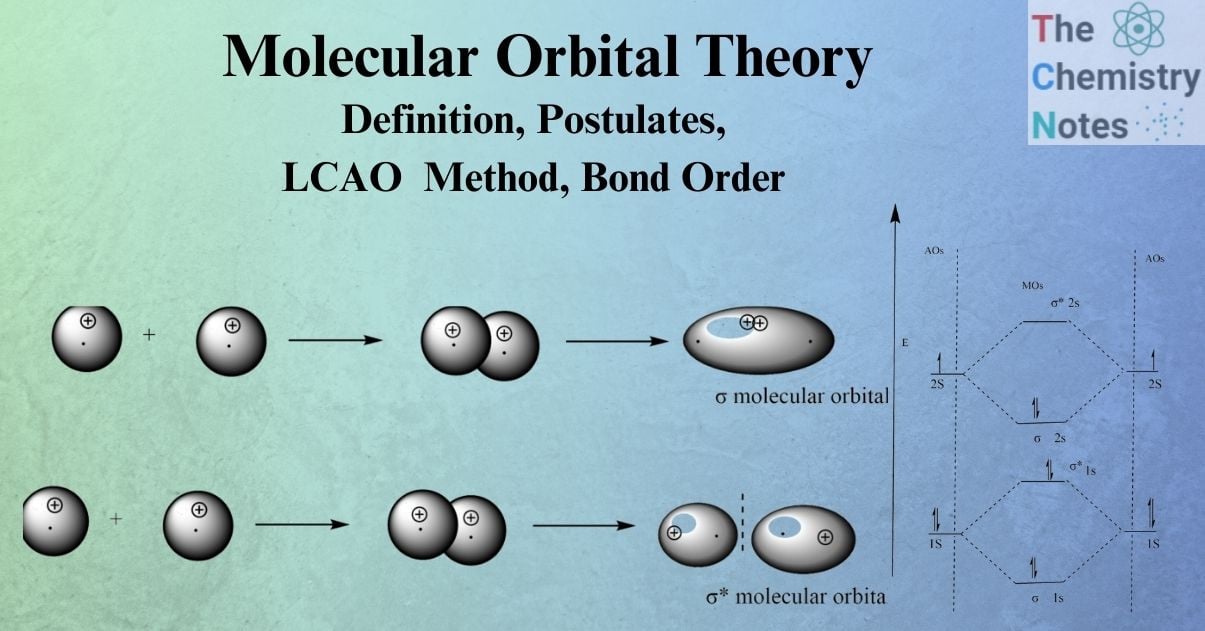
S-S overlap
An atom’s s -atomic orbital combines with another atom’s s orbital to form σ bonding and σ antibonding ( σ*) molecular orbitals.
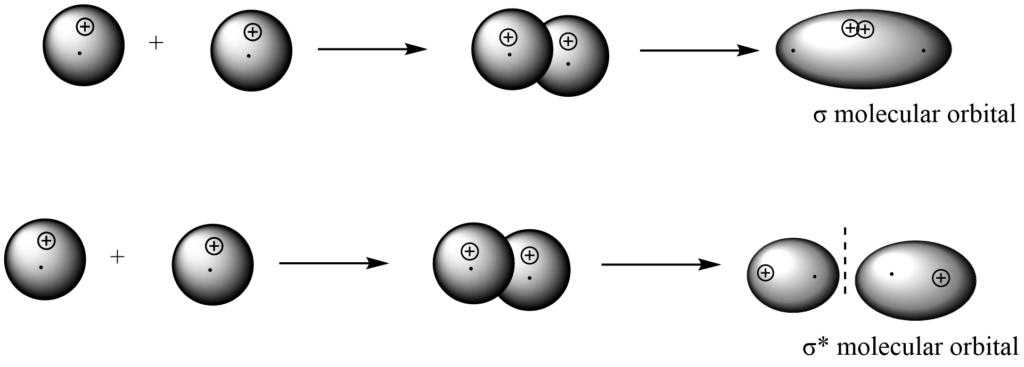
S – Px overlap
An atom’s s -atomic orbital combines with another atom’s px-orbital (X-axis considered as molecular axis) to form σ bonding and σ antibonding molecular orbitals.
Bonding MO is formed by addition overlap (with the same sign), whereas anti-bonding MO is formed by subtraction overlap (with the opposite sign).
Bonding MO is formed by addition overlap (with the same sign), whereas anti-bonding MO is formed by subtraction overlap (with the opposite sign).
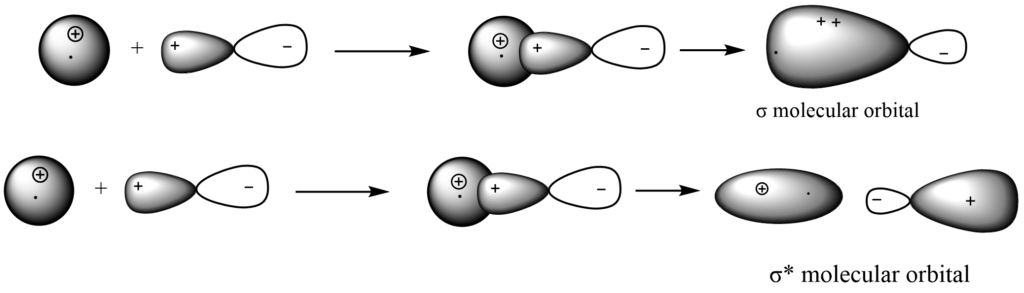
P-P overlapping
The p- orbitals are denoted by the letters PX, Py, and Pz. Three types of overlaps are therefore feasible.
i. PX – PX overlap
To create a bonding molecule orbital (by addition overlap) and an anti-bonding molecular orbital (by removal overlap) concurrently, two p atomic orbitals with similar energies overlap (mix).
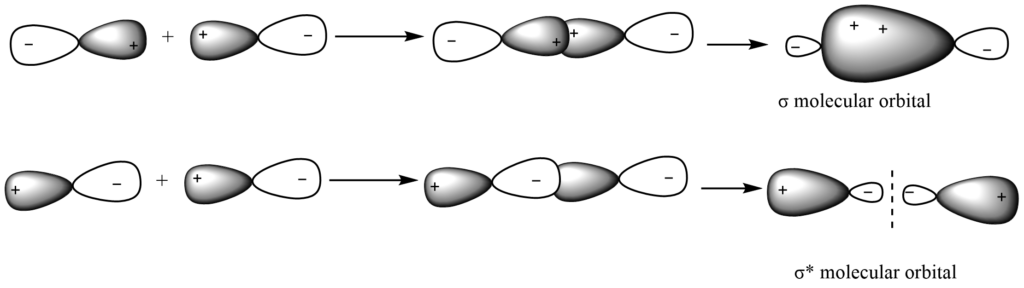
ii. Py– Py overlap
Two Py -atomic orbitals having comparable energy mix to produce π bonding molecular orbital (by addition overlap) and π antibonding (π *) molecular orbitals (by subtraction overlap)
simultaneously.
Non-bonding overlapping
The atomic orbitals overlap to generate bonding and antibonding molecular orbitals. When two AOs undergo addition overlap, a bonding MO with lower energy is generated, indicating that the MO is stabilized. However, the subtraction overlap of two atomic orbitals produces an anti-bonding molecular orbital with high energy. In other words, the MO is unstable.
However, there is a third type of overlap that neither stabilizes nor destabilizes, in which the two atomic orbitals experience addition and subtraction overlap simultaneously.
In this overlap, an orbital with a + or — lobe combines with both the (+) and (—) lobes of another orbital. As a result, the stabilization caused by the overlapping of (+) with the (+) lobe is destabilized by an equivalent amount of overlapping of (+) with (—). Non-bonding molecular orbitals are those with such type of overlapping where there is no change in the energy of the orbital. If any electrons are still present, they are left as lone pairs in such orbitals.
Conditions for LCAO
It is important to remember that any two atomic orbitals will not necessarily combine to form molecular orbitals. The atomic orbitals must follow some criteria to form molecular orbitals.
1. Atomic orbitals must have comparable energy
The energy of atomic orbitals involved in the formation of MO should be the same or nearly similar. So, when the homonuclear diatomic molecule like X2 is formed (for example, H2, N2, O2), It is feasible for 1s – 1s and 2s – 2s to overlap. However, crossover between 1S – 2s and 1s – 2p is impossible. However, in the case of hetero-nuclear diatomic molecules like AB (e.g., HCl, HF, etc.), this type of overlap is to be expected.
2. The atomic orbitals must significantly overlap.
“The greater the extent of overlap of AOs, the greater the electron density between the two nuclei.” This requirement is frequently referred to as the maximum overlap principle.
3. The atomic orbitals must have the same symmetry with respect to the molecular axis
It is not necessary that two atomic orbitals having comparable energy will combine to form molecule orbitals. he AOs must have the same symmetry (orientation) about the molecular axis in order to generate MOs.
Features of molecular orbital theory
- According to molecular orbital theory, all of the atomic orbitals of combining atoms first mix to generate a new set of orbitals known as molecular orbitals. Once molecular orbitals form, combining atoms lose their atomic orbital identity.
- The number of molecular orbitals produced is equal to the total number of combining atomic orbitals.
- The molecular orbitals are formed by the linear combination of atomic orbital (LCAO).
- Addition and subtraction overlap of atomic orbital produces bonding and antibonding molecular orbitals respectively.
- Molecular orbitals have different energy, size, and shape.
- The shape of the molecular orbital generated is determined by the shape of the intermixing atomic orbitals.
- The electron present in the molecular orbital is represented by its wave function Ψ and Ψ2 represents the probability of finding electron density in the molecular orbitals.
- The electrons present in the molecular orbitals must follow the Aufbau principle, Pauli’s exclusion principle, and Hund’s rule.
Bond order
The number of electrons in bonding and antibonding molecular orbitals can be seen in the filled molecular orbital diagram. The net contribution of electrons to a molecule’s bond strength is calculated by determining the bond order that arises from electrons filling in molecular orbitals. We define bond order as the number of electron pairings between two atoms when utilizing Lewis structures to describe the distribution of electrons in molecules. A single bond, for example, has a bond order of one, a double bond has a bond order of two, and a triple bond has a bond order of three. When using the molecular orbital model of electron distribution, we define bond order differently, although the resulting bond order is usually the same. Although both methods describe the same phenomenon, the Molecular orbital theory is more precise and can deal with conditions in which the Lewis structure method fails.
According to the molecular orbital model, an electron contributes to a bonding interaction if it is located in a bonding orbital and to an antibonding interaction if it is situated in an antibonding orbital. The bond order is determined by subtracting the destabilizing (antibonding) electrons from the stabilizing (bonding) electrons. We divide by two to obtain the bond order since a bond consists of two electrons.
Bond order = Total electrons in bonding MO — Total electrons in antibonding / 2
= (Nb— Na) / 2
Features of bond order
- The bond order of a diatomic molecule is equal to the number of bonds formed between two atoms. For example, the bond order of the O2 molecule is two. Therefore, the O2 molecule is written as O = O.
- A molecule’s stability, bond energy, and bond dissociation energy are strongly associated with its bond order.
- Bond order is inversely proportional to the molecule’s bond length.
- The molecular structure with zero bond order indicates that it is unstable or non-existent.
Magnetic properties
The presence or absence of unpaired electrons in molecular orbitals determines a molecule’s or molecular ion’s magnetic characteristics. Paramagnetic molecules are those which contain one or more unpaired electrons, whereas diamagnetic molecules are those without any unpaired electrons. The magnetic moment can be determined using the relationship:
Magnetic moment μ = √n(n+2) B.M
where n= number of unpaired electrons
Molecular orbital diagrams and stability
I. H2 molecule
Electronic configuration of hydrogen (H) = 1S1
Total number of electrons in H2 molecule = 2
Molecular orbital electronic configuration of H2 molecule = (σ 1S)2 , (σ* 1S)0
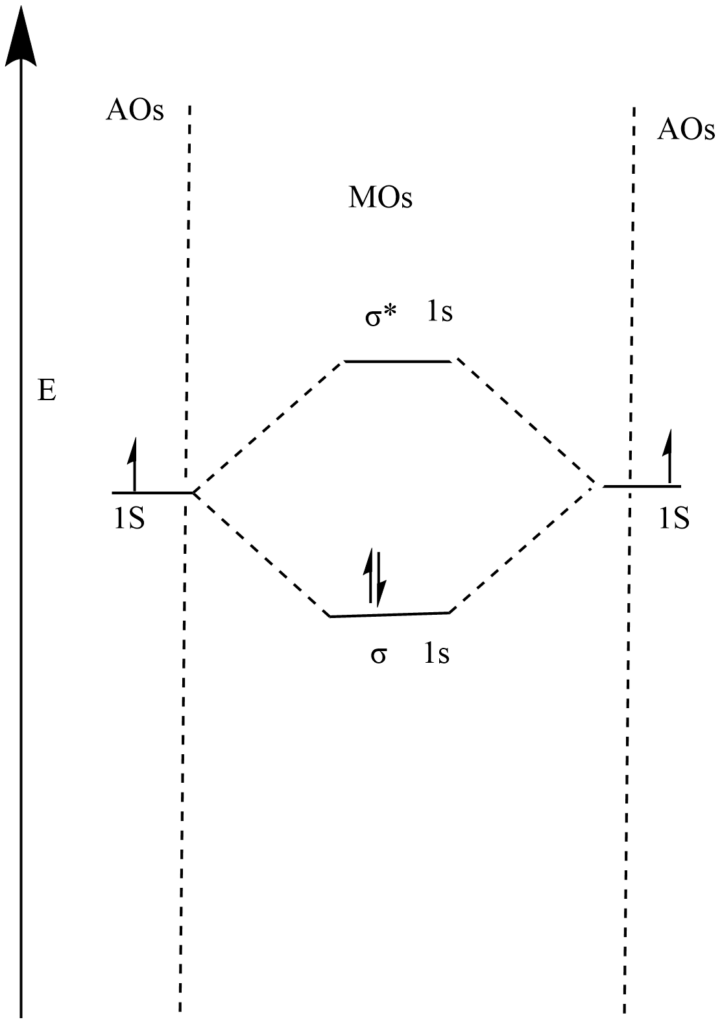
Molecular orbital diagram of H2 molecule
The two electrons first occupy a lower energy level i.e. σ 1S molecular orbital.
so bond order = (Nb— Na) / 2
= 2 – 0 / 2
= 1
i.e., a single bond binds two H-atoms to form an H2 molecule.
Since there are no unpaired electrons in molecular orbitals an H2 molecule is diamagnetic.
II. Li 2 molecule
Electronic configuration of Lithium (Li) = 1S2, 2S1
Total number of electrons in Li2 molecule = 3 x 2 = 6
Molecular orbital electronic configuration of Li2 molecule = (σ 1S)2 , (σ* 1S)2 , (σ 2S)2
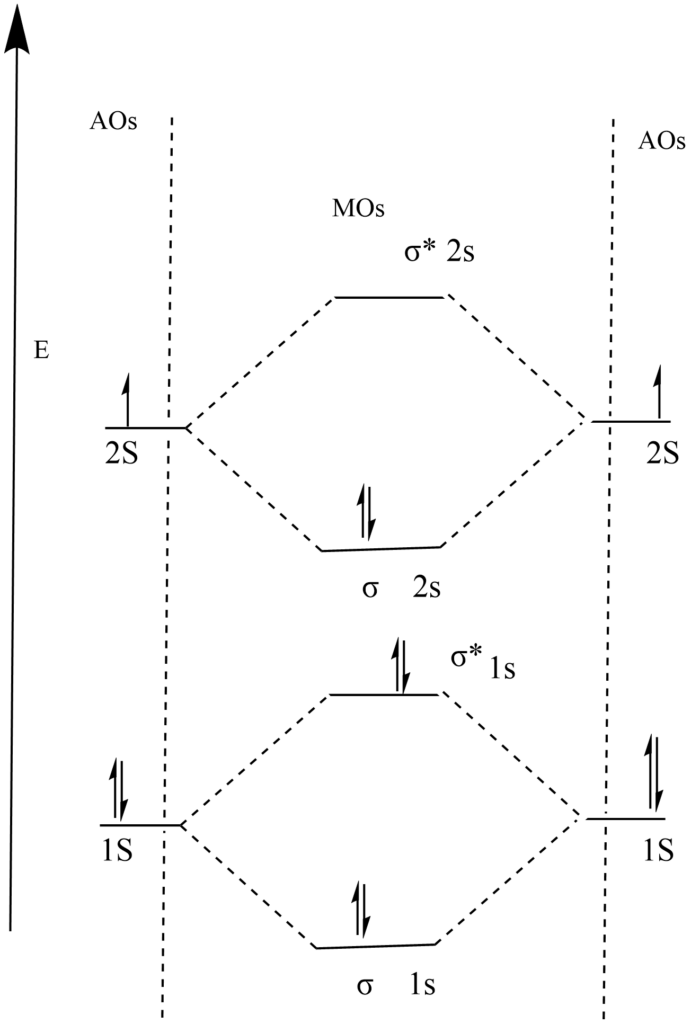
Molecular orbital diagram of Li2 molecule
bond order = (Nb— Na) / 2
= 4 – 2 / 2
= 1
Lithium exists as a Li2 molecule in the vapor state.
Since all the molecular orbitals contain paired electrons it is diamagnetic.
III. Be 2 molecule
Electronic configuration of Lithium (Be) = 1S2, 2S2
Total number of electrons in Be2 molecule = 4 x 2 = 8
Molecular orbital electronic configuration of Be2 molecule= (σ 1S)2 , (σ* 1S)2 , (σ 2S)2 , (σ* 2S)2
bond order = (Nb— Na) / 2
= 4 – 4 / 2
= 0
Be2 has a bond order of zero. It represents that it does not exist.
Suggested video:
References
- Lee, J.D. (1996) Concise Inorganic Chemistry. 5th Edition, Chapman and Hall Ltd., London, 619-621.
- https://byjus.com/jee/molecular-orbital-theory/.
- https://chemed.chem.purdue.edu/genchem/topicreview/bp/ch8/mo.html.
- https://www.chem.fsu.edu/chemlab/chm1046course/motheory.html.
- https://chem.libretexts.org/Bookshelves/General_Chemistry/Chem1_(Lower)/09%3A_Chemical_Bonding_and_Molecular_Structure/9.08%3A_Molecular_Orbital_Theory.
- https://www.ch.ic.ac.uk/vchemlib/course/mo_theory/main.html.
- https://www.chem.uci.edu/~unicorn/old/H2A/handouts/PDFs/LectureB6.pdf.
- https://ocw.mit.edu/courses/5-111sc-principles-of-chemical-science-fall-2014/bceb89367a2924eab97dcb476cec369e_MIT5_111F14_Lec13.pdf.
