Molecular compounds, occasionally called covalent compounds, exhibit a broad array of physical properties due to the different types of intermolecular attractions such as different kinds of polar interactions. When non-metal atoms share a pair of electrons, Molecular compounds are formed. Depending on the extent of covalent bonding in a compound, this can take two different structures: either simple molecular compounds or giant covalent (or macromolecular) structures.

Properties of Molecular Compounds
- Intermolecular forces, or the forces between different molecules of the same substance, are responsible for many of the properties of molecular compounds. Molecular compounds have weak intermolecular forces. These are distinct from the very powerful intramolecular forces, which are created by the covalent bonds that hold the molecules’ atoms together.
- The temperature at which a substance changes from a solid to a liquid is known as the melting point. Molecular compounds typically have low melting points because of the weak intermolecular forces that govern them.
- The temperature at which a substance changes from a liquid to a gas is known as the boiling point. Because it does not require much energy to separate and vaporize molecules, weak intermolecular forces also contribute to the low boiling points of molecular compounds.
- Because of their low melting and boiling points, molecular substances are typically gases and liquids at room temperature. At room temperature, some molecular substances are solids, but they are soft and flexible.
- Solubility refers to a substance’s ability to dissolve into a solute, such as water. Molecular compounds have a low solubility. Because they are usually nonpolar, they do not dissolve well. Other polar molecular substances dissolve better than nonpolar molecular substances because water is a polar molecule with positive and negative ends.
- The ability to conduct electricity is referred to as conductivity. Molecular compounds have no ions and are electrically neutral. They are poor conductors of electricity because they have no charge and no free electrons. Instead, molecular compounds are excellent insulators.
Simple Molecular Compounds Structure
A simple molecule is made up of a small number of atoms that are held together by strong covalent bonds. Simple molecules can exist as elements (such as iodine or nitrogen) or compounds (such as water or ammonia).
Crystals can also form from substances with simple molecular compounds structure, such as iodine. This reflects the regular packing of molecules in a lattice structure. Ice also forms a crystalline lattice. Because of hydrogen bonding, ice and water have unusual properties.
The distance between the nuclei of adjacent iodine molecules is greater than the distance between the nuclei of the iodine molecule itself. This is due to the fact that the forces between molecules are weak van der Waals forces, whereas the forces between atoms within the molecule are strong covalent bonds. To overcome the weak van der Waals forces between the molecules, very little energy is required. When iodine crystals are heated, the lattice is easily broken down because iodine has a low melting point.
The melting and boiling points of compounds with simple molecular structures are low. When the compound is solid, the simple molecules that make up the compound form a regular lattice that is held together by weak intermolecular forces. Because these interactions are so weak, little energy is required to overcome them, resulting in simple molecular structures that are usually gaseous or liquid at room temperature.
![Iodine molecules [Simple Molecular Compounds Structure]](https://scienceinfo.com/wp-content/uploads/2023/01/image-53.png)
Giant Molecular Compounds Structures
Some covalently bonded structures have a three-dimensional network of covalent bonds that extends the entire length of the structure. These structures are referred to as giant molecular compounds structure or giant covalent structures.
Because of the large number of strong covalent bonds connecting the entire structure, they have high melting and boiling points. Because of the large number of strong covalent bonds involved, it takes a significant amount of energy to break them apart. Both elements (graphite and diamond) and compounds (silicon dioxide) can form massive molecular structures. Carbon and graphite are two different forms of the same element. Allotropes are various crystalline or molecular forms of the same element.
Graphite
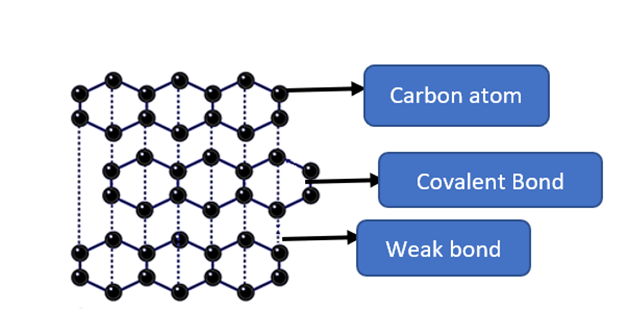
Carbon atoms are arranged in planar layers in graphite. Carbon atoms are arranged in hexagons within the layers. Strong covalent bonds connect each carbon atom to three other carbon atoms. Each carbon atom’s fourth electron occupies a p orbital. Each carbon atom’s p orbitals in each planar layer overlap sideways. Above and below the plane of the carbon rings, a cloud of delocalized electrons forms. These electron clouds combine to form extended delocalized electron rings. The carbon atom layers are held together by weak van der Waals forces.
Properties of Graphite
Graphite’s properties are related to its structure.
- High melting and boiling points: there is strong covalent bonding between carbon atoms throughout the layers. Overcoming these strong bonds requires a lot of energy.
- Graphite is soft and easily scratched. The forces between the carbon atom layers are weak. When a force is applied to the graphite layers, they can slide over each other. The layers easily flake away. Because of this ‘flakiness,’ graphite is used in pencil ‘leads’ and feels slippery.
- Good electrical conductor: when a voltage is applied, delocalized electrons (mobile electrons) can move along the layers.
Uses of Graphite
- Today’s pencil lead is actually the safer graphite that most people are familiar with.
- The modern world relies on the portable energy source that batteries provide. Graphite is an important component of modern batteries because it serves as a lithium-ion host for the negative electrodes. Because of its incredible conductivity due to the presence of free electrons, graphite is the most preferred and cost-effective material.
- Graphite has been and continues to be an important material for use in both historical and modern nuclear reactors due to its extreme purity and ability to withstand high temperatures and pressure.
- Because of its ability to conduct electricity while dissipating or transferring heat away from critical components, the crystalline form of graphite is one of the most commonly used in the electrical industry.
- Graphite is used in refractories as a counter-electrode in arc furnaces due to its high thermal resistance properties and mechanical stability at such high temperatures.
- Graphite electrodes and connecting pins are used in the production of steel in electric arc furnaces. It is used as a protective agent for steel ingots and for the metallurgical furnace lining, and powdered graphite is used as a carbon raiser to give steel its strengthening properties.
- Graphene is essential in the production of sports equipment because it works with other layers of material to add super strength, responsiveness, durability, and agility.
Diamond
Diamond is a mineral made entirely of carbon. It is the most popular gemstone and the hardest naturally occurring substance known. Diamonds have a variety of important industrial applications due to their extreme hardness.
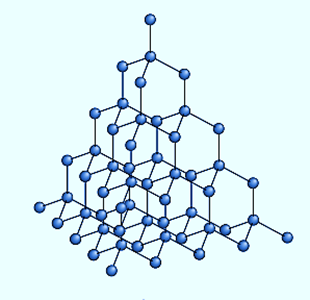
Each carbon atom in diamond forms four covalent bonds with another carbon atom. The carbon atoms are arranged tetrahedrally around each other. The network of carbon atoms runs almost entirely through the structure. Diamond has a crystalline structure due to the regular arrangement of its atoms.
Properties of Diamond
Diamond properties are related to their structure.
- High melting and boiling points: covalent bonding is strong throughout the structure. It takes a lot of energy to break these strong bonds and separate the atoms.
- Diamond is difficult to scratch because the three-dimensional network of strong covalent bonds is difficult to break.
- Each of the four outer electrons on every carbon atom is involved in covalent bonding, so it does not conduct electricity or heat. This means that there are no available free electrons to carry the electric current.
Uses of Diamonds
- Diamonds are most commonly used in jewelry. They frequently embed rings, necklaces, bracelets, and earrings. Diamonds are extremely hard and durable, making them ideal for use in rings, necklaces, and other jewelry. They are also very sparkly, which makes them popular for use in engagement rings and other visible jewelry.
- Also used to decorate other items such as watches, chandeliers, and vases.
- They are also used in a wide range of electronic devices. Because they are very durable and can withstand a lot of heat, they are used in computer chips and other electronic components.
- Diamonds are also used in lasers because they can produce a very powerful light beam.
- They have a variety of industrial applications. Because they can cut through most materials, they are used in drills and saws.
- Diamonds are also used in turbines due to their ability to withstand extremely high temperatures.
Silicon (IV) oxide
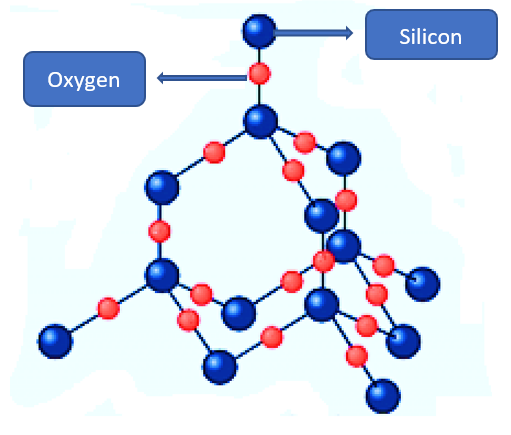
There are various types of silicon(IV) oxide. The structure of the silicon (IV) oxide found in the mineral quartz is similar to that of diamond.
Each silicon atom has four oxygen atoms bonded to it, but each oxygen atom has only two silicon atoms bonded to it. SiO2 is the formula for silicon (IV) oxide. Silicon dioxide has properties that are similar to diamond. It crystallizes into hard, colorless crystals with high melting and boiling points and does not conduct electricity.
Properties of Silicon (IV) oxide
- In its pure form, silicon(IV) oxide is a colorless crystalline solid.
- This oxide is a macromolecular compound in which the oxygen and silicon atoms are covalently linked in what are known as tetrahedral basic units. These basic units are arranged in crystobalite in the same way that diamond units are arranged, whereas in quartz and tridynamite they are arranged in a spiral form around an axis. Silicon(IV) oxide is nonvolatile, owing largely to its structure. It is also hard, in contrast to carbon(IV) oxide, which is a gas at room temperature.
- This oxide melts at approximately 1500oC. When cooled, it solidifies into a glass-like solid known as fused silica (quartz glass), which has a very low coefficient of expansion and is therefore very heat resistant. Furthermore, fused silica is acid-resistant and is frequently used in the manufacture of laboratory apparatus.
- Except for hydrogen hexafluorosilicates, silicon(IV) oxide is insoluble in water and all acids (IV)
- This compound dissolves in hot concentrated potassium or sodium hydroxide solution to form the corresponding trioxosilicates(IV) and water as an acid oxide.
- When heated strongly with metallic salts, it can displace more volatile acid oxides to form the corresponding trioxosilicates(IV). The displaced oxides typically escape as vapors or gases. Silicon(IV)oxide is not a volatile substance.
- At very high temperatures, it also reacts with carbon to form silicon carbide, SiC. Because of its hardness, the carbide produced is used as an abrasive.
Uses of Silicon (IV) oxide
- Powdered quartz is used in the production of silicon carbide, silicon tetrafluoride, furnace linings, silica bricks, and trioxosilicates (IV).
Fused silica is used in the manufacture of optical lenses and prisms, scientific instruments, and heat-resistant articles. Fine, strong threads of fused quartz are used to suspend component parts in electrical instruments. - This sand-like compound is widely used in the manufacture of brick, mortar, enamel, and glass.
- Quartz crystals are used to control radio frequency transmitters precisely. Because large quartz crystals are transparent to ultraviolet light, they are used for lenses in optical instruments such as binoculars and telescopes.
- Kieselguhr readily absorbs liquids and is used as an absorbent for nitroglycerine, an explosive, in the manufacture of dynamite. Kieselguhr is used in medicine to make antiseptic dry dressings.
Watch out: Properties of Simple Molecular Compound Structure and Giant Molecular Compound Structure
References
- Whitten, Kenneth W.; Gailey, Kenneth D.; Davis, Raymond E. (1992). “7-3 Formation of covalent bonds”. General Chemistry (4th ed.). Saunders College Publishing. p. 264. ISBN 0-03-072373-6.
- https://sites.google.com/site/ellesmerealevelchemistry/module-2-foundations-in-chemistry/2-2-electrons-bonding-and-structure/2-2-2-bonding-and-structure/2-2-2-n-simple-molecular-lattices?pli=1
- https://studymind.co.uk/notes/properties-of-covalent-structures-2/
- https://chem.libretexts.org/Courses/Saint_Francis_University/CHEM_113%3A_Human_Chemistry_I_(Muino)/04%3A_Molecular_Compounds/4.05%3A_Characteristics_of_Molecular_Compounds
- https://www.bbc.co.uk/bitesize/guides/zgq8b82/revision/2#:~:text=Diamond%20and%20graphite%20are%20different,of%20their%20properties%20are%20different.
- Giant covalent structures: diamond and graphite-Jeffrey H Williams https://doi.org/10.1088/978-1-6817-4625-8ch9
- https://study.com/learn/lesson/molecular-compound-properties-overview.html