A mixture is made up of one or more pure components with different ratios. Mixtures can be either heterogeneous or homogeneous. When compared to homogeneous mixtures, heterogeneous mixtures can be visually divided into individual components. The most prevalent kind of homogeneous mixture is a solution, which can be a solid, liquid, or gas.
Interesting Science Videos
What is Mixture?
When two or more chemicals mix without undergoing a chemical change, the resulting substance is known as a Mixture. Combinations of two or more different types of substances are known as mixtures.
Chemical components and compounds, as well as other substances, are mechanically blended or mixed to create mixtures.
The result of the mixing of things retains its uniqueness and is not chemically mixed.
Properties of Mixture
- The components of a mixture may be easily separated,
- Each component retains its original properties.
- The proportion of the components is also variable.
Types of Mixtures
There are two types of Homogeneous Mixture and Heterogeneous Mixture.
Homogeneous Mixture
Homogeneous mixtures are those in which the components are evenly distributed throughout the mixture. For instance, salt and water are a homogenous mixture since any amount of water will taste the same when sipped. This demonstrates that the salt is dispersed evenly throughout the mixture.
Characteristics of Homogeneous Mixture
- These blend’s ingredients are evenly distributed throughout.
- The components cannot be separated using centrifugal force.
- The Tyndall effect, or the scattering of light by the medium’s particles when a light beam is impacted onto it, does not occur in homogeneous mixtures. The light beam is scattered, making the light’s path apparent.
- Its particle size is about 1 nm.
Heterogeneous Mixture
Heterogeneous mixture are those in which the components are dispersed unevenly across the combination and do not have a uniform distribution throughout the mixture. Sand and water, for instance, are an example of a heterogeneous mixture since sand does not disperse evenly in water. Sand with water, sugar and salt, ice in water, etc. are a few examples.
Characteristics of Heterogeneous Mixture
- A heterogeneous mixture has components that are not evenly distributed throughout it.
- Just by glancing at the combination, you can draw a line separating the components.
- The size of the particles ranges from 1 nm to 1 m.
- The Tyndall effect may be seen in them.
Interesting Facts about Mixture
- Smoke is a gaseous mixture of particles suspended in the air.
- Tap water contains a combination of water and other particles. Distilled water is commonly defined as pure water or H2O.
- Many of the substances we come into contact with on a daily basis are mixtures, including the air we breathe, which is a mixture of gases such as oxygen and nitrogen.
- Blood is a mixture that can be separated into two main parts by a machine called a centrifuge: plasma and red blood cells.
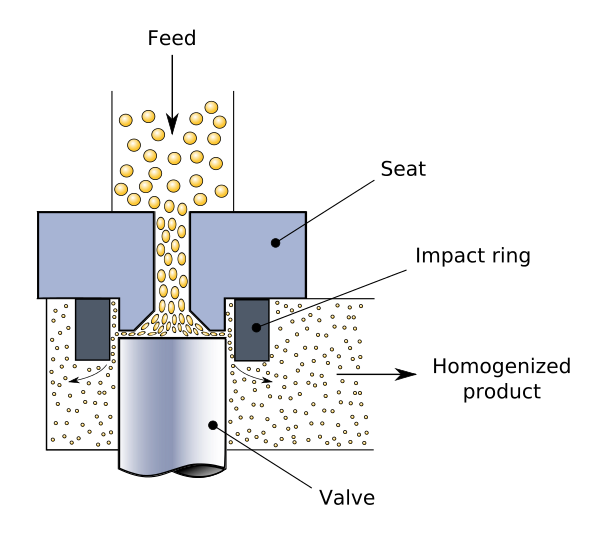
Homogenization
Homogenization, also known as homogenisation, is any of several techniques used to make a mixture of two mutually insoluble liquids uniform throughout. This is accomplished by converting one of the liquids into a condition consisting of extremely minute particles scattered uniformly across the other liquid.
Milk homogenization is a common example, in which the milk fat globules are decreased in size and distributed uniformly throughout the rest of the milk.
References
- Whitten K.W., Gailey K. D. and Davis R. E. (1992). General chemistry (4th ed.). Philadelphia: Saunders College Publishing. ISBN 978-0-03-072373-5.
- Petrucci, Ralph H.; Harwood, William S.; Herring, F. Geography (2002). General chemistry: principles and modern applications (8th ed.). Upper Saddle River, N.J: Prentice Hall. ISBN 978-0-13-014329-7. LCCN 2001032331. OCLC 46872308.
- De Paula, Julio; Atkins, P. W. (2002). Atkins’ Physical Chemistry (7th ed.). ISBN 978-0-19-879285-7.
- Ashworth, William; Littl1, Charles E., eds. (2001). “Mixture”. The Encyclopedia of Environmental Studies
- https://www.geeksforgeeks.org/what-is-a-mixture/
- https://byjus.com/chemistry/mixtures/#1
