Metallic bonding, a type of chemical bonding, gives metals their distinctive properties, such as malleability, luster, and conductivities for both heat and electricity. Hence, metallic bonds are critical to metal properties. Metallic bonds differ from covalent and ionic bonds because it is formed by the attraction of positively charged metal nuclei and their delocalized valence electrons between two or more metal atoms.
What is Metallic Bond?
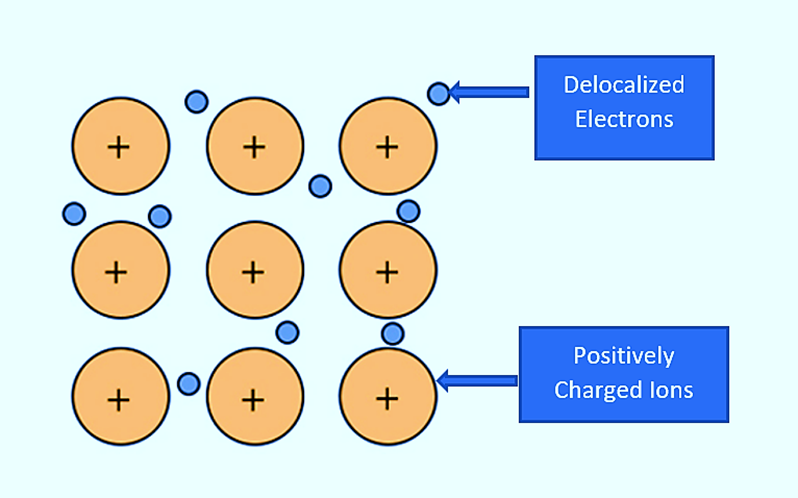
Metallic bonds are chemical bonds that hold metal atoms together. The metallic bond is the force of attraction between these free-moving (delocalized) electrons and positive metal ions.
They differ from covalent and ionic bonds in that the electrons are delocalized, that is, they are not shared by only two atoms. On the other hand, electrons in metallic bonds float freely through the lattice of metal nuclei. Because metallic bonds are not discrete directional bonds between specific atoms, it is more common to refer to metallic “bonding” rather than individual bonds.
What is Metallic Bonding?
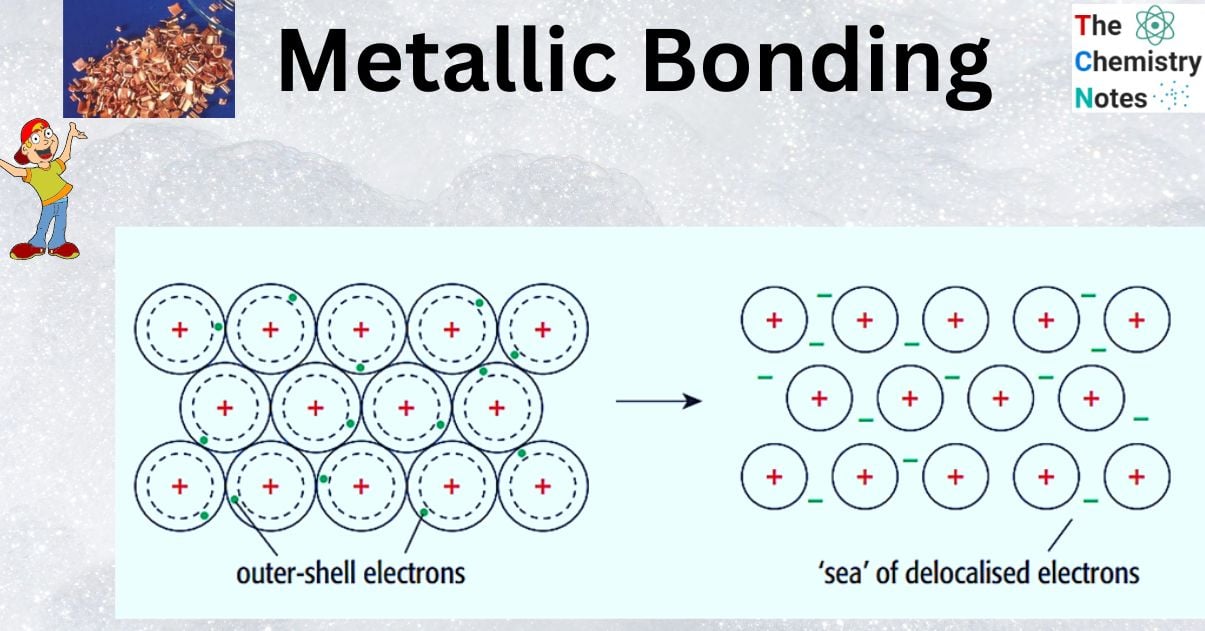
Metallic bonding is a chemical bonding that occurs as a result of the attraction of delocalized electrons and positive ions. Metal is made up of its delocalized electrons and numerous positive metal ions arranged in a regular pattern packed closely together.
Metals with this type of bonding have many distinct material properties, such as excellent thermal and electrical conductivity, high melting points, and malleability.
- The atoms in a metal are packed closely together in a regular arrangement known as a lattice.
- In a lattice, metal atoms lose their outer shell electrons and become positive ions.
- The electrons in the outer shell occupy new energy levels and are free to move throughout the metal lattice.
- These electrons are referred to as delocalised electrons (mobile electrons). Delocalised electrons are electrons that are not attached to any specific atom or bond.
How Do Metallic Bonds Form?
Metal atoms’ outer energy levels (the s and p orbitals) overlap. At least one valence electron in a metallic bond is not shared with a neighboring atom and is not lost to form an ion. Instead, the electrons form a “electron sea” in which valence electrons can freely move from one atom to the next.
- According to the electron sea model, all metal atoms contribute their valence electrons to form a “sea” of electrons. Metals’ physical properties are due to their delocalized electrons.
- Metal valence electrons are only loosely bonded to their nuclei because they are shielded by electrons with higher inner energy levels.
- Valence electrons in a metallic bond, in other words, can be associated with any atom in the metal sample.
- This, combined with the atoms’ closely packed and patterned lattice-like arrangement, allows valence electrons to roam free from a specific parent atom and its corresponding nucleus.
- The electrons in this sea or cloud have a negative collective charge and are electrically attracted to the positively charged nuclei, forming an omnipresent or blanket of cohesion.
Strength of Metallic Bonding
Metallic bonding is extremely strong. This is due to the strong electrostatic attraction between the ions’ positive charges and the negative charges of the delocalized electrons. This electrostatic attraction works in all directions.
Metallic bonding strength increases as follows:
- Increasing positive charge on the ions in the metal lattice: The greater the positive charge on the ions in the metal lattice. The stronger the bond, the more strongly it attracts delocalized electrons.
- Decreasing size of metal ions in the lattice: As metal ion size decreases, the strength of metallic bonding increases. The shorter the distance between the positive nucleus at its center and the delocalized electrons around it, the smaller the radius of the cations. As a result, their electrostatic forces of attraction are stronger.
- Increasing number of mobile electrons per atom: The greater the number of mobile electrons per atom, the more electrons each atom can contribute to the sea of electrons that holds the lattice together.
Metallic Bonding and Physical Properties of Metals
The bonding of a metal determines its physical properties. The arrangement of ions and electrons in a compound determines whether or not it can conduct electricity, as well as its melting and boiling points.
- High melting and boiling points of Metals
It takes a significant amount of energy to weaken the strong attractive forces between metal ions and delocalised electrons. Only at high temperatures can these attractive forces be overcome. Mercury, on the other hand, is a liquid at room temperature. This is because some of the electrons in a mercury atom are more tightly bound to the nucleus than usual, weakening the metallic bonds between atoms.
- Metals are good conductors of electricity
An electric current flows through a piece of metal when a voltage is applied to it because the delocalised electrons (mobile electrons) are free to move. Metallic bonding is the only type of bonding that reliably predicts whether or not a solid will conduct electricity. Covalent solids cannot conduct electricity because none of their electrons are free to move, with the exception of graphite. Ionic solids cannot conduct because their electrons and ions are not free to move.
- Metals are good conductors of heat
The movement of delocalised electrons and the vibrations passed on from one metal ion to the next contribute to heat conduction.
- Metals are ductile and malleable.
Positive ion layers can slide over one another and take on different positions.
Metallic bonding is not broken because the outer electrons do not belong to any specific metal atom, so the delocalised electrons move with them. As a result, metallic bonds are not broken, and metals are strong but flexible. They can be hammered and bent into various shapes, as well as drawn into wires, without breaking.
- Metallic luster
When light strikes a metallic surface, the photon’s energy is absorbed by the sea of electrons that makes up the metallic bond. Energy absorption excites electrons, increasing their energy levels. These excited electrons quickly return to their ground states while emitting light. This light emission caused by electron de-excitation gives the metal a shiny metallic luster.
Metallic Bonding Examples
Metallic Bonding in Sodium
The electronic structure of sodium is 1s22s22p63s1. When sodium atoms come together, the electron in one sodium atom’s 3s atomic orbital shares space with the corresponding electron on a neighboring atom to form a molecular orbital, much like a covalent bond.
The difference is that each sodium atom is touched by eight other sodium atoms – and the sharing occurs between the central atom and all of the eight other atoms’ 3s orbitals. And each of these eight is in turn touched by eight sodium atoms, which are in turn touched by eight atoms – and so on and so forth, until you reach the end.
All of the 3s orbitals on all of the atoms overlap, resulting in a large number of molecular orbitals that extend across the entire piece of metal. Of course, because each orbital can only hold two electrons, there must be a massive number of molecular orbitals. The outer electrons have delocalised throughout the metal structure. This means they are no longer attached to a specific atom or pair of atoms, but can be thought of as freely moving throughout the structure.
As a result, the outer electrons of each atom are involved in this delocalisation or sea of electrons. The nucleus and inner electrons of each atom are essentially sodium ions, Na+. The strong forces of attraction between the delocalised electrons and the positive ions hold the metal together.
The softness and low melting point of sodium can be explained by the relatively low number of electrons in the electron sea and the relatively small charge on the sodium cation. However, when compared to the alkali metal magnesium, the electron sea here contains twice as many electrons as the one in sodium (since two 3s electrons are delocalized into the sea). Because of the greater magnitude of charge and the higher electron density in the sea, magnesium has a much higher melting point (650oC) than sodium.
Metallic Bonding in Magnesium
The outer electronic structure of magnesium is 3s2. Because both of these electrons become delocalised, the “sea” has twice the electron density of sodium. Because the remaining “ions” have twice the charge, “ions” and “sea” will be more attracted to one another. In reality, each magnesium atom has 12 protons in its nucleus, compared to 11 for sodium. The nucleus is screened from delocalised electrons in both cases by the same number of inner electrons – the 10 electrons in the 1s2 2s2 2p6 orbitals.
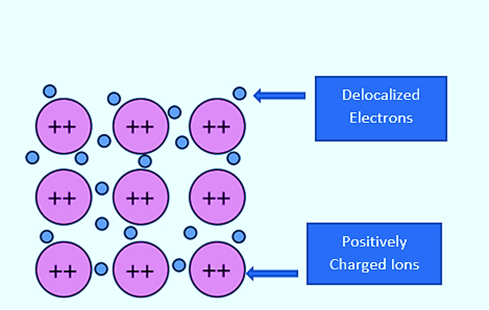
That means the net pull from the magnesium nucleus will be 2+, but only 1+ from the sodium nucleus. As a result, not only will there be more delocalised electrons in magnesium, but the net pull from the magnesium nuclei will also attract them more.
Because magnesium atoms have a slightly smaller radius than sodium atoms, the delocalised electrons are closer to the nuclei. In addition, each magnesium atom has twelve near neighbors as opposed to eight for sodium. Both of these factors contribute to the bond’s overall strength.\
Frequently Asked Questions (FAQ)
How strong is a metallic bond?
Atoms in metals are highly attracted to one another. that takes a lot of energy to go through that. Because of this, metals have high boiling points, with tungsten (5828 K) being especially high.
What is the sea of electrons model?
According to the “electron sea” concept of metallic bonding, cations are fixed points inside a moving “sea” of electrons.
What is a metallic bond and how does it form?
Metallic bonds develop in materials when the charge is spread out over an area larger than a single atom. On the periodic table, left-handed elements like zinc and copper most frequently form metallic connections. Because metals are solid, their atoms are tightly packed in a predictable arrangement.
What does the electron sea model explain?
The electron sea model provides an explanation for many of the physical characteristics of metals. They function well as electrical conductors because they allow electrons to travel around easily. They are flexible because of the freely moving electrons and how easily the cations slide past one another. They reflect light because of the free electrons.
What is the Difference Between Metallic Bonding and Ionic Bonding?
Ionic bonds are formed by the exchange of electrons between two chemical species. They are caused by an imbalance in the electronegativities of the linked atoms. Metallic bonds, on the other hand, are produced when a solid, defined lattice of metal cations shares a sea of delocalized valence electrons. Both of these modes of bonding, however, involve electrostatic forces of attraction.
Is NaCl a metallic bond?
NaCl does not have a metallic bond. An ionic link is responsible for the formation of NaCl. Metallic bonding occurs between metal atoms within the same metal substance.
How does the metallic bond account for transition metals’ higher melting and boiling points than alkali metals?
The total number of valence electrons and the charge on the nucleus determine the strength of the metallic bonding. The strength of the metallic bond grows as the number of valence electrons and charges increases. Because of this, alkali metals are soft with low melting and boiling temperatures, whereas transition metals are hard with high melting and boiling points.
References
- Shriver and Atkins’ Inorganic Chemistry. Oxford University Press. 2010. pp. 74–. ISBN 978-0-19-923617-6.
- Structure and Bonding in Crystalline Materials , pp. 326 – 362 DOI: https:// doi.org/10.1017/CBO9780511816116.009
- https://studymind.co.uk/notes/properties-of-metallic-bonding/
- https://study.com/learn/lesson/metallic-bond-examples-characteristics-strength.html
- https://www.chemguide.co.uk/atoms/bonding/metallic.html
- https://www.wondriumdaily.com/metallic-bonding-and-covalent-bonding-properties-and-differences/
- https://byjus.com/chemistry/metallicbonds/#:~:text=What%20is%20a%20Metallic%20 Bond,several%20positively %20charged%20metal% 20ions.
- https://chemistrytalk.org/metallic-bonding/
- https://www.expii.com/t/metallic-bond-formation-compounds-8645
