Metal ion indicators are the organic compounds used in the complexometric titration. Different types of metal indicators are used for the different metal ions. These indicators are usually used to detect the endpoint of EDTA titration. The indicator changes its color when the metal ion is bound with the indicator.
Metal ion indicator forms a complex with a metal ion.
M 2+ + HIn2− → Min− + H+
Where HIn2− represents indicators formed at a particular pH. However, metal ion indicator complexes are general metal-EDTA complexes. The indicator point and shows a change in color.
Examples of metal ion indicator
- Patton and Reeder’s indicator (12-14 pH) is used for the determination of ca.
- Murexide ( 10 – 11 pH range) is used for the determination of Cu, Ni, Co, Ca etc.
- Solochrome black ( 10 pH) is used for the determination of Hg, Mn, An, Cd, Mg, Pb, and Ca.
- Xylenol orange (pH 1 – 2) is used for the determination of Bi, Th, Zn, Co
(pH 4 – 6) for Cd, Pb, Sn, Ni, Mn
5. Methyl thymol blue (pH 0-2) used for Th. Hf, Hg, Zn, Co, Cd
(pH 4-6) used for Al, Ni, Mn
pH 12 for Ca, Sr, Ba, Mg
Requirement/ criteria for metal ion indicator (for use in the visual detection of the endpoint)
- At various pH levels, they should have a distinct color.
- The color reaction needs to be either selective or specific.
- The metal-ion indicator complex needs to be sufficiently stable; otherwise, dissociation prevents the observation of a sharp change.
- IV. The metal EDTA complex would have to be more stable than the meta ion indicator complex. Otherwise, it is impossible to see good observation.
- V. The color contrast between the metal ion indicator complex and the free indicator should be such that it should be easy to observe.
- VI. The indicator must be metal ion sensitive for color changes to take place as close to the corresponding point as is reasonably achievable.
- VII. The above condition must be fulfilled within the pH range that the titration is being conducted.
Erichrome Black T ( a metal ion indicator)
Erichrome black T is a metal ion indicator that is used to precisely determine the end point in a complexometric titration. It is also known as solochrome black, and it is often employed as an indicator in complexometric titration.
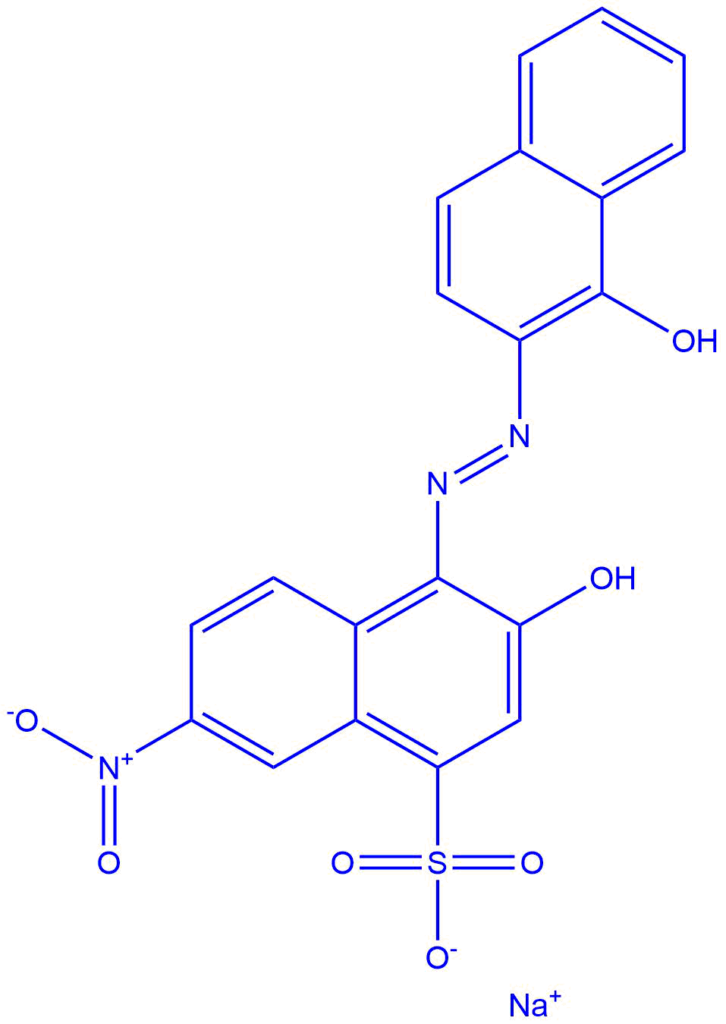
It is sodium 1- (1 – hydroxyl – 2- naphthyl azo – 6- nitro – 2- naphthol -4 -sulfate. It shows two sensitive regions of color change and exhibits acid-base behavior. The role of Erichrome black T is to carry out metal ion titration using EDTA in complexometric titration.
The indicator first creates a colored complex with metal ions that are stronger than it. The EDTA then replaces the indicator and forms a metal-EDTA complex, leaving the indicator free of a different color. When all of the indicators are moved by EDTA and the end point is observed,obtained a color change is observed.
M + In → M -In Complex
M -In Complex + EDTA → M-EDTA complex + In
Advantages of Erichrome Black T (EBT)
- Aqueous solutions of the indicator are stable almost independently.
- It functions as an acid-base indicator.
H3In ⇌ H2In – (Low pH) ⇌ HIn– – (pH 5.7-7.3) ⇌ In3- (pH 10.5 -12.5)
Bright red Clear blue Yellow reddish orange
- The acidic properties pf hydroxy groups are expressed by Pk1= 8.14 and Pk2= 13.35
- The blue color of calmagite at pH =10 is changed to red by addition of Mg- ion. The change being reversible,
HIn– – + Mg 2+ ⇌ Mg In– (red)
suggested video
References
- https://biocyclopedia.com/index/chem_lab_methods/types_of_complexometric_titration.php
- https://chrominfo.blogspot.com/2023/02/metal-ion-indicator-in-complexometric.html
- D.A, Skoag, Principle of Instrumental Analysis, (3rd Edition), Saunders collage publishing, 1985.
- http://www.csun.edu/~hcchm003/321/321120313.pdf
