The mass spectrometer is generally designed to perform three basic functions, they are:
- To vaporize the compounds of various volatility.
- To produce ions from the neutral compounds in the vapor phase.
- To separate the compounds according to their mass-to-charge ratio and record them.
Instrumentation of Mass Spectrometry (MS)
Sample handling system
The sample handling system is used to introduce the sample into the ion source at low pressure. Heating is required for less volatile sample. Solid, liquid, and gas samples can be used in mass spectrometry.
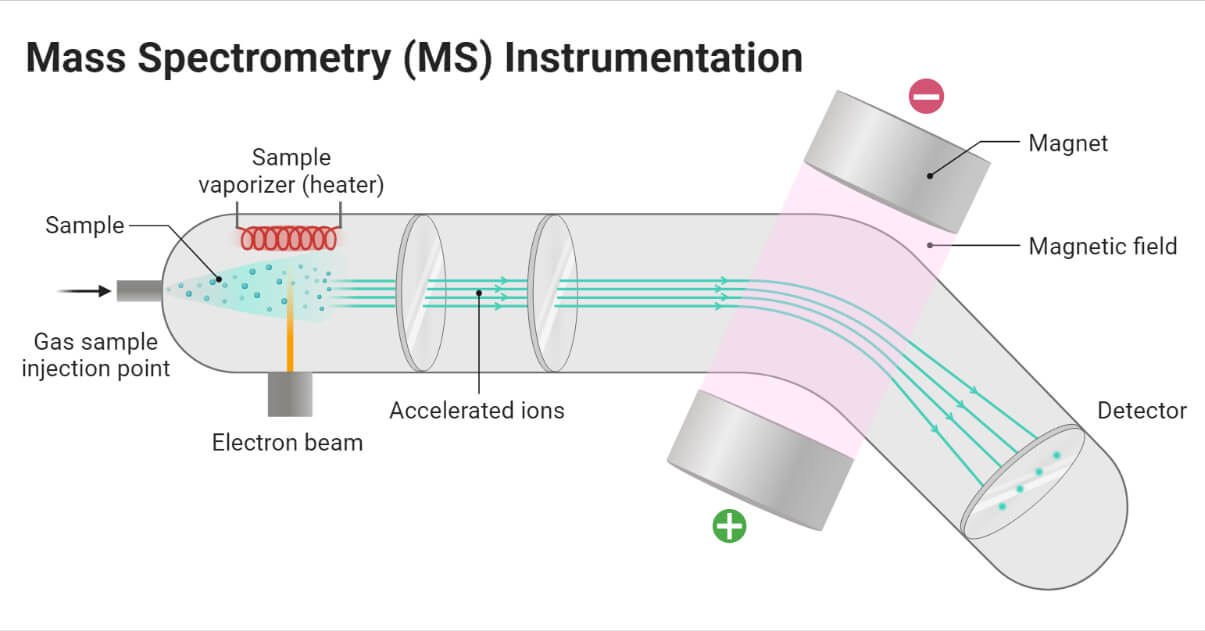
Figure: Instrumentation of Mass Spectrometry (MS), Created with biorender.
Ionization process in Mass Spectrometry
Samples are ionized by removing one or more electrons to give the molecular ions and fragment ions. Different ionization methods are used in mass spectroscopy. They are
Electron ionization/Electron impact
This is the oldest and best-characterized method of ionization. In this process, the beam of electrons passes through the gas sample. The electrons collide with the neutral molecule and knock off another electron to form molecular ions or fragment ions.
Chemical ionization
The chemical ionization process involves reagent gas like methane, isobutane or ammonia. First, the reagent gas is ionized by electron impact, and the reaction between reagent gas ions and the sample produces the ions of the analyte.
Field desorption
In this process, the sample is placed on the anode of pair of electrodes, and the electrodes are placed at too high a potential. SO, the sample desorption occurs to produce molecular and quasimolecular ions with sufficient internal energy for extensive fragmentation.tt
Atmospheric pressure chemical ionization (APCI)
In APCI, a corona discharge is used to ionize the sample in the atmospheric region. The gas phase ionization in APCI is more effective for analyzing less-polar species.
Fast atom bombardment (FAB)
It involves the bombarding of samples by using beams of xenon atoms of high transitional energy. In this process, xenon atoms are first ionized by using the electrons to give xenon radical cations. The xenon radical cations are accelerated to 6-10 keV to give radical cations of high transitional energy. The radical cations are then passed through xenon to form the high-energy xenon atom Xe and Xe.+ions are removed by the electric field.
The compounds of interest are placed in a metal sheet and ionized by a high-energy beam of a xenon atom.
Electrospray ionization
The electrospray ionization involves placing the ionizing voltage across the nebulizer needle present at the outlet from high-performance liquid chromatography. ESI can produce the multiply charged ions with the number of charged tending to increase with an increase in molecular weight. This is popularly used for water-soluble biomolecules like protein, peptides, and carbohydrates.
Matrix-assisted laser desorption ionization
In this process, the sample is dissolved in the solution containing an excess of a matrix like dihydroxy benzoic acid, and sinapic acid, which consists of chromophores, capable of absorbing laser light. When the solution is placed in the laser light the solution absorbs the laser light and produces plasma, which results in ionization and vaporization of the sample.
Acceleration of ions
Ions are accelerated to attain the same kinetic energy. The ions pass through three accelerating slits with decreasing voltage.
Deflection of ions
Ions are deflected by using the magnetic field on the basis of their m/z ratio. The lighter ions with a high positive charge are more deflected than others.
Detection of ions
Detectors detect the ions passing through the mass analyzer on the basis of the m/z ratio.
Mass analyzers of Mass Spectrometry
Different mass analyzers are:
Quadrupole mass filter
Qurdupole mass filter consists of four cylindrical rods arranged in squares parallel to the direction of the ion beams. In this radiofrequency and direct current are applied. The combination of Rf and direct current generates the oscillating electrostatic field between the region of ions. When the ions enter into the mass analyzer depending upon the ratio of Rf and Dc voltage oscillating electrostatic field will be generated for ions. Ions with an inappropriate m/z ratio will undergo an unstable oscillation and hit the rods. But ions with the correct m/z ratio undergo stable oscillation and strike the detector to give a signal.
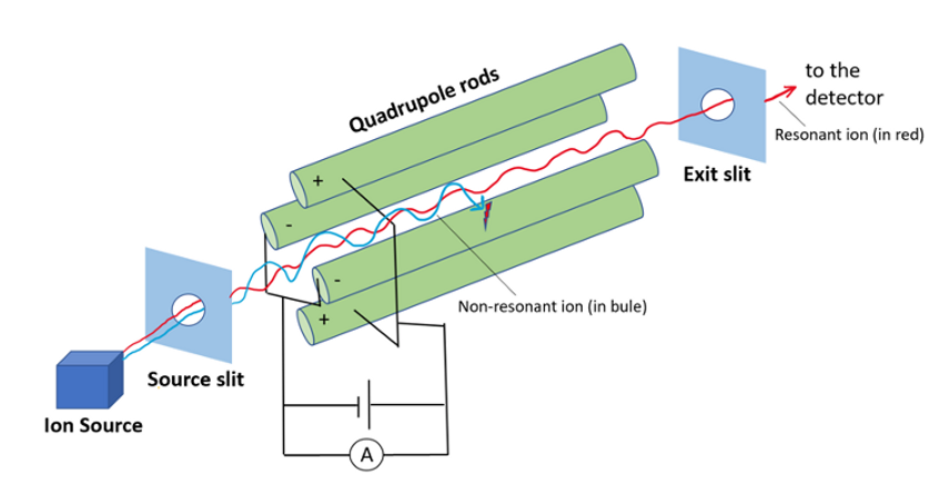
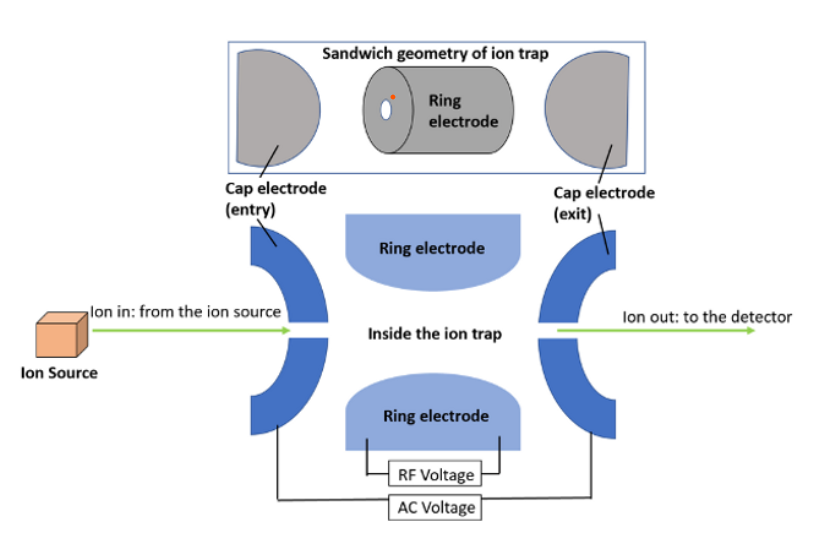
Quadruple ion trap:
It is the spherical configuration of a linear mass filter. The difference between a linear mass filter and an ion trap is that the linear mass filter directs sorted ions through the detector, whereas the ion trap temporarily retains unsorted ions within the trap. After scanning the electric field, they are released to the detector. Because of their speed and sensitivity, quadrupole ion trap methods are well suited for use with capillary gas chromatography. These are compact, inexpensive, and easy to use.
Time of flight mass spectrometer
In this procedure, ions are subjected to a high potential difference (V) to attain the same transitional energy in electron volt. They are further accelerated and transferred to the field-free region. There they are separated overtime on the basis of their m/z values. This is simply based on the principle that the velocities of two ions depend on the mass of ions. In this process, ions should be created at the same instant and they should have the same kinetic energy. The lighter ions have higher velocity compared to the higher ions, so on passing towards the detector, they hit the detectors first.
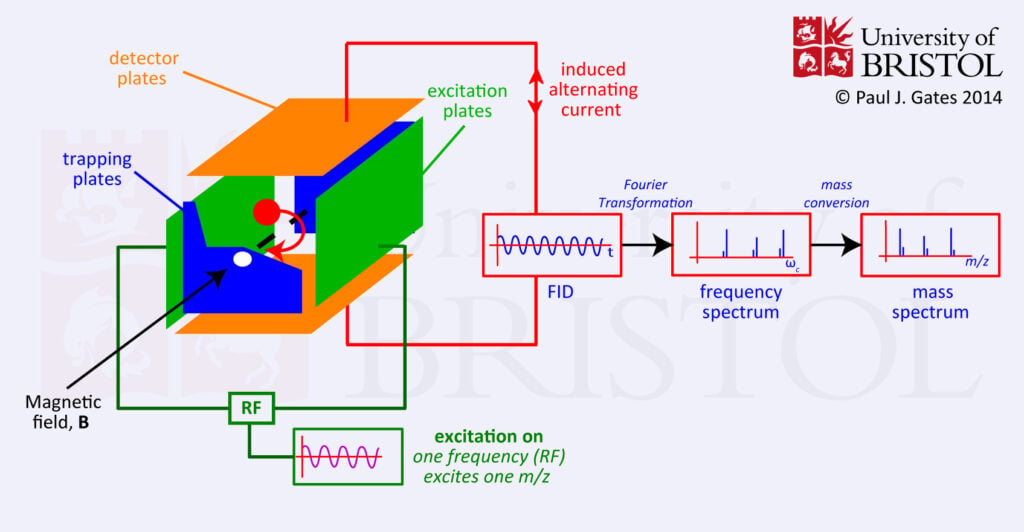
Fourier transform-Ion Cyclotron Resonance
Ions, generated by the electron beam from heated filament are passed into cubic cells under vacuum conditions. An electric tapping potential and a magnetic field hold these ions in place. Depending on their m/z ratio, each ion assumes the cycloidal orbit at its own characteristic frequency. By varying the electric field, these frequencies are scanned until the cycloidal frequency is in resonance with the applied radio frequency. The motion of ions with the same frequency is coherent at resonance, and a signal is detected.
Detectors of Mass Spectrometry
Photoplate detectors:
It is the earliest and the largest ion detector. It involves the dispersion of ions along the lengthy focal plane resulting in adequate unit mass resolution across the entire spectral region. So photoplate detector make able to simultaneous detection and signal integration.
Electron multipliers
The ions emerging from the mass analyzer are detected using an electron multiplier. It is a common type of ion detector that comes in a variety of designs. In this detector, ions striking the conversion dynode generate secondary ions. The dynodes then generate the electron cascade effectively multiplying the single incident ions by 106 or more. The degree of multiplication is determined by the individual dynode surface composition, acceleration per stage (bias voltage), the number of dynodes (6-20), and bias current circuit design.
Photomultiplier
Ions strike the photomultiplier detector, resulting in electron emission. The emitted electron then strikes the phosphor screen, giving rise to photons. After that, the photons are amplified by passing through the multiplier. The advantage of this detector is that it operates in a vacuum state, which prevents contamination and extends the detector’s lifetime.
Faraday cups
The name faraday cup is named after Michael Faraday. In this detector, a metal cup is placed in the path of the ion beam and is connected to an electrometer that measures the current of the ion beam. When the incident ions strike the dynode surface, it produces electrons and induces a current, which is amplified and recorded. Faraday cups are able to detect the higher current which cannot be measured by an electron multiplier.
Photographic plates
These are the time integrating devices generally used with the radiofrequency spark instruments. Photographic plates integrate the ion signal over time and detect the ions of all masses simultaneously. It is the simplest and oldest type of ion detector
For more details on the instrumentation of mass spectroscopy you can watch this video.
References
- Silverstein, R. M., Webster, F. X., & Kiemle, D. J. (2005). Spectrometric identification of organic compounds. Hoboken, NJ: John Wiley & Sons.
- https://byjus.com/chemistry/mass-spectrometry/
- https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/spectrpy/massspec/masspec1.htm
- https://www.technologynetworks.com/analysis/articles/types-of-ion-detector-for-mass-spectrometry-347890
- Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Instrumental_Analysis/Mass_Spectrometry/Mass_Spectrometers_(Instrumentation)/Mass_Analyzers_(Mass_Spectrometry)
- https://microbenotes.com/mass-spectrometry-ms-principle-working-instrumentation-steps-applications/
- https://www.priyamstudycentre.com/2022/02/mass-spectrometry.html
- https://www.chemguide.co.uk/analysis/masspec/howitworks.html
- https://barclayphysics.fandom.com/wiki/How_are_the_ions_produced_and_detected
- https://pubs.acs.org/doi/pdf/10.1021/ac053495p
- https://www.researchgate.net/publication/328661548_Mass_Spectrometry_Detectors_Review

