Manganese is the chemical element that belongs to the group 7 of period 4 in the periodic table. It is a transition metal with the atomic number 25 and is represented by the symbol ‘Mn’. In nature, this element is not present as a free element but rather in mixtures with iron and other minerals.
In terms of abundance in the crust of the Earth, manganese is second only to iron among the transition elements; its physical and chemical characteristics are comparable to those of iron, with the exception that it is harder and more brittle. Manganese is a trace mineral that the body contains in very small levels.
History of Manganese
- The name manganese derives from the latin word ‘magnes’ meaning magnet.
- Long used as a pigment, manganese dioxide is a substance which is common in nature. The mineral form of MnO2 pigments were used to create old cave paintings in Gargas around 30,000–24,000 years ago.
- Egyptian and Roman glassmakers used manganese compounds to either enhance or remove the color from glass.
- Glass from Venice dating back to the 14th century shows evidence of its continued use as “glassmakers soap” throughout the Middle Ages and into modern times.
- In the 1700s, several chemists made unsuccessful attempts to separate the metal from pyrolusite.
- Johann Heinrich Pott, a glass technician from Berlin, conducted a chemical analysis of it in 1740 and demonstrated that it did not contain iron as had been supposed. He was able to create potassium permanganate (KMnO4), one of the most potent oxidizing agents known to man, from it.
- In the year 1774, Gottlieb Gahn, the Swedish chemist and mineralogist was the first person to do so.
- However, Ignatius Kaim, a student at Vienna, had already written about how he had made manganese metal in his 1771 dissertation.
- It was discovered in 1816 that added manganese makes iron stronger.
Occurrence of Manganese
- The crust of the Earth contains large amounts of manganese in combination with other elements. It is the 12th most abundant element on the Earth’s crust. Land-based resources are abundant but unevenly dispersed.
- About 80% of the world’s known manganese resources are in South Africa; other important manganese deposits are in Ukraine, Australia, India, China, Gabon, and Brazil. Manganese dioxide (MnO2), the most common compound of manganese, makes up about 0.14% of the Earth’s crust.
- Manganese nodules also known as polymetallic nodules,(concretions of manganese with some iron, silicon, and aluminum) are believed to cover large areas of the ocean floor.
- Manganese in the nodules is estimated to be substantially higher than the land reserves. These nodules are composed of 29% manganese content.
- Manganese ores usually consist of dark brown to black oxides particularly pyrolusite (MnO2) and psilomelane [(Ba,H2O)2Mn5O10]. Manganese carbonate (rhodochrosite, MnCO3) and silicate (braunite, MnSiO3) may occur locally. South Africa, Australia, China, Gabon, Brazil, India, Kazakhstan, Ghana, Ukraine, and Malaysia are the primary mining locations for manganese.
- All species require the element manganese. It is accumulated by several species, including diatoms, mollusks, and sponges. Although generally, they contain approximately 1 ppm, fishes can have up to 5 ppm and mammals can have up to 3 ppm in their tissue.
Isotopes of Manganese
The only stable isotope of manganese (Mn) that is found naturally is 55Mn. 18 radioisotopes have been identified, with 53Mn, 54Mn, and 52Mn having the longest half-lives at 3.7 million years, 312.3 days, and 5.591 days, respectively.
All of the remaining radioactive isotopes have half-lives that are less than 3 hours and the majority of these have half-lives that are less than 1 minute.
| Isotope | Natural abundance (atom %) |
|---|---|
| 55Mn | 100 |
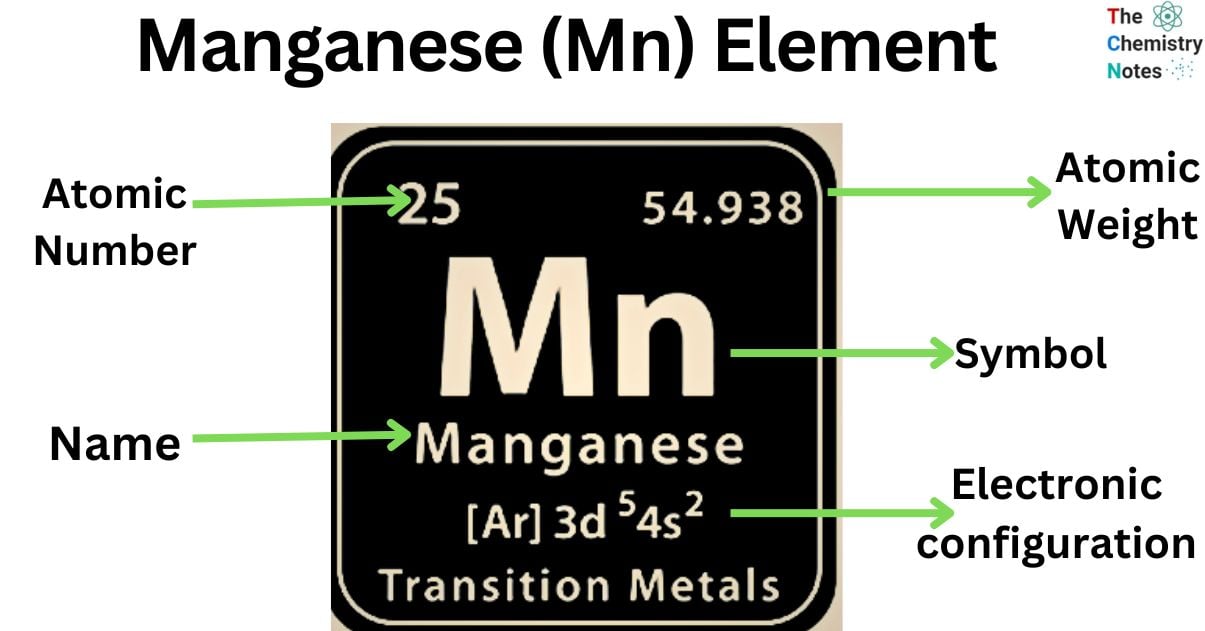
Elemental Properties of Manganese
| Electronic Configuration | [Ar] 3d5 4s2 |
| Atomic Number | 25 |
| Atomic Weight | 54.9380 g mol -1 |
| State at 20°C | Solid |
| Group, Period, and Block | 7, 4, d-block |
| Density | 7.3 g cm-3 at 20°C |
| Ionic radius | 0.08 nm (+2) ; 0.046 nm (+7) |
| Van der Waals radius | 197 pm |
| Electron shells | 2, 8, 13, 2 |
| Electrons | 25 |
| Protons | 25 |
| Neutrons in most abundant isotope | 30 |
Physical Properties of Manganese
- Manganese is a brittle, hard, shiny, steel-gray metal.
- Manganese has a melting point of 1,246°C (2,275°F) and a boiling point of around 2,061°C (3,742°F).
- Its density is 7.30 grams per cubic centimeter.
- As the temperature rises, the element transforms from one form to another.
- It is not malleable, in fact, it is so brittle, that it cannot be machined(cutting, bending, and shaping of metal using mechanical means) in its pure form.
- It is a poor conductor of electricity and heat.
- Manganese is ferromagnetic, meaning it is attracted to magnets.
- Manganese is also resistant to corrosion. This makes it a valuable material for use in corrosion-resistant.
| Color/physical appearance | grayish-white |
| Melting point/freezing point | 1246°C, 2275°F, 1519 K |
| Boiling point | 2061°C, 3742°F, 2334 K |
| Density | 7.3 (g cm−3) |
| Malleability | No |
| Ductility | No |
| Electronegativity | 1.55 (Pauling Scale) 1.75 (Allen Scale) |
Chemical Properties of Manganese
- Manganese is a moderately active metal. It combines slowly with oxygen in the air to form manganese dioxide (MnO2 ).
- At higher temperatures, it reacts more rapidly. It may even burn, giving off a bright white light.
- Manganese reacts slowly with cold water, but more rapidly with hot water or steam.
- It dissolves in most acids with the release of hydrogen gas.
- It also combines with fluorine and chloride to make manganese difluoride (MnF2) and manganese dichloride (MnCl2 ).
Chemical Reaction of Manganese
- Reaction of manganese with air
Despite being a slightly more electropositive than its neighbors in the periodic table, manganese is not very reactive to air. Small amounts of surface oxidation occur on lumps of manganese. When finely separated, manganese metal burns in the air. It burns in oxygen to form the oxide Mn3O4 and in nitrogen to form the nitride Mn3N2.
3Mn (s) + 2O2 (g) → Mn3O4 (s)
3Mn (s) + N2 (g) → Mn3N2 (s)
- Reaction of manganese with water
Manganese does not react with water under normal conditions.
- Reaction of manganese with the halogens
Manganese burns in chlorine (Cl) to form manganese(II) chloride, MnCl2.
Mn (s) + Cl2( g) → MnCl2 (s)
Manganese also reacts with bromine (Br) or iodine (Id) to form manganese(II) bromide, MnBr2, or manganese(II) iodide, MnI2 respectively.
Mn(s) + Br2(g) → MnBr2(s)
Mn(s) + I2(g) → MnI2(s)
The fluorides manganese(II) fluoride (MnF2) and manganese(III) fluoride (MnF3) are produced by the equivalent reaction between the metal and fluorine, F2.
Mn (s) + F2 (g) → MnF2 (s)
2Mn (s) + 3F2 (g) → 2MnF3 (s)
- Reaction of manganese with acids
Manganese metal quickly dissolves in diluted sulfuric acid to create solutions that contain the ionized Mn(II) and hydrogen gas, H2. In practice, the Mn(II) is present as the virtually colorless complex ion [Mn(OH2)6]2+.
Mn(s) + H2SO4(aq) → Mn2+(aq) + SO42-(aq) + H2(g)
Uses of Manganese
- Steel alloys use up to 90% of the manganese that is produced. By melting and combining two or more metals, an alloy is created. The characteristics of the combination are distinct from those of the individual metals. Steel becomes harder and more resilient to rusting, mechanical stress, and corrosion when manganese is added. Aluminum alloys containing 1.5% manganese are used to make beverage cans in order to increase corrosion resistance.
- Ferromanganese (About 48% of the manganese in this alloy is mixed with iron and carbon) is the most commonly used manganese alloy. A very wide range of steel items, including tools, heavy equipment, railroad tracks, bank vaults, building materials, and automobile parts, are made from ferromanganese as the raw material.
- Important non metallurgical uses include battery cathodes, soft ferrites used in electronics, micronutrients in fertilizers, micronutrients in animal feed, water treatment chemicals, colorant for automobile undercoating, bricks, frits, glass, textiles, and tiles.
- It serves as a catalyst and is a component of the original dry-cell battery.
Biological Uses of Manganese
- Manganese is a vital mineral that the body needs in order to operate effectively. It can be found in food including leafy green vegetables, whole grains, and nuts.
- It engages in a variety of chemical processes in the body, including those that break down protein, carbs, and cholesterol. It could possibly have a role in bone growth.
- Manganese aids in the formation of bones, connective tissue, blood clotting components, and sex hormones in the body. Additionally essential for healthy nerve and brain function is manganese.
- People use manganese for manganese deficiency. It is also used for hay fever, osteoporosis, osteoarthritis, wound healing, and many other conditions, but there is no good scientific evidence to support these uses.
- In plants, manganese functions as an activator and co-factor of several metalloenzymes. Mn plays a significant part in a wide variety of enzyme-catalyzed events, including redox reactions, phosphorylation, decarboxylation, and hydrolysis, due to its capacity to easily change oxidation state in biological systems.
- The Mn-dependent germin enzyme oxalate oxidase (OxOx) catalyzes the oxygen-dependent breakdown of oxalate by oxidation into two moles of CO2, in a process that also results in the production of H2O2.
- Manganese (II) is the most common oxidation state of Mn in plants, and it exhibits quick ligand exchange kinetics. Because Mg(II) has similar ion properties and needs to the ligand environment of the metal binding sites, it can frequently replace Mn.
- Symptoms of the Mn deficiency develop as interveinal chlorosis in newly emerged leaves, while prolonged deficiency causes leaf necrosis, which appears as brown spots between veins in older leaves.
Health Effects of Manganese
- Manganese is one of the chemical elements that affect living things in both beneficial and detrimental ways. For plants and animals to remain healthy, a very small quantity of the element is required. The manganese is used by enzymes in an organism. An enzyme is a molecule that makes chemical reactions occur more quickly in cells. If manganese is missing from the diet, enzymes do not operate efficiently. When cells start to die, an illness develops in the organism.
- Humans ingest manganese mostly through food, such as spinach, tea, and herbs. Foods including grains and rice, soy beans, eggs, almonds, olive oil, green beans, and oysters have the largest quantities of this element. Manganese is absorbed by the body and then circulated through the blood to the liver, kidneys, pancreas, and endocrine glands.
- Due to its high level of toxicity, exposure to manganese dust and fumes should be limited to no more than five milligrams per cubic meter (mg/m3), even for brief periods of time.
- Having too much manganese might have negative health effects. These issues include exhaustion, emotional difficulties, weakness, and even paralysis. The only way to receive such a large dose is in a factory or mine. Workers may inhale manganese dust in the air.
- Low body manganese levels can cause infertility, distorted bones, weakness, and convulsions.
Osteoporosis
A number of trace elements, such as vanadium and boron, as well as manganese, are essential for healthy bones. Although there is no concrete proof that manganese can prevent osteoporosis, one study revealed that postmenopausal women who took calcium, zinc, copper, and manganese supplements saw decreased spinal bone loss. Osteoporosis can affect anyone, but it most frequently affects older women. Up to 50% of all women and 25% of men over 50 will break a bone as a result of osteoporosis.
Environmental Effects of Manganese
- Manganese compounds can be found in nature as solids in soil and tiny particles in water. Humans increase the amount of manganese in the air by burning fossil fuels and engaging in industrial activities. Surface water, groundwater, and sewage water can all contain manganese from human sources. Manganese will enter soils through the use of manganese insecticides.
- Manganese compounds exist naturally in the environment as solids in the soils and small particles in the water. Manganese particles in air are present in dust particles. These usually settle to earth within a few days.
- In case of animals, manganese is an essential component of over thirty-six enzymes used for the metabolism process. Animals who consume insufficient amounts of manganese will experience difficulties with proper bone development, growth, and reproduction. Because the lethal dose for some species is quite low, they have limited chance of surviving even lower manganese concentrations when these surpass the critical dose. Manganese-containing compounds can disrupt the liver, lungs, and blood vessels, lower blood pressure, prevent animal fetuses from developing normally, and harm the brain.
Watch out the video to learn some interesting information related to Manganese.
References
- Los Alamos National Laboratory (2001), Crescent Chemical Company (2001), Lange’s Handbook of Chemistry (1952), CRC Handbook of Chemistry & Physics (18th Ed.) International Atomic Energy Agency ENSDF database (Oct 2010)
- https://www.chemicool.com/elements/manganese.html
- Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- https://www.thoughtco.com/manganese-facts-606557
- https://www.webelements.com/manganese/chemistry.html
- https://www.lenntech.com/periodic/elements/mn.htm
- https://www.webmd.com/vitamins/ai/ingredientmono-182/manganese
- Manganese in Biological Systems Ebany J. Martinez-Finley, Sudipta Chakraborty & Michael Aschner : https://doi.org/10.1007/978-1-4614-1533-6_284
- The Biochemical Properties of Manganese in Plants Sidsel Birkelund Schmidt* and Søren Husted doi: 10.3390/plants8100381

