Several plants contain indicators, the most common of which is litmus, which is collected from lichens. It is one of the most basic and earliest pH indicators.
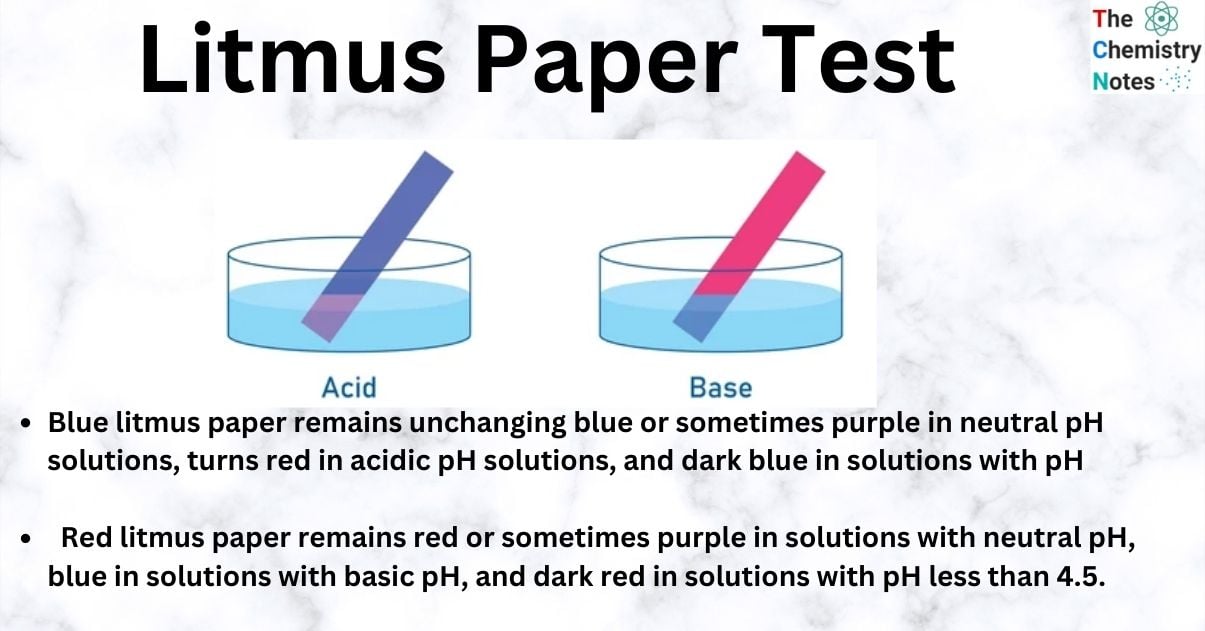
In the litmus paper test, if the substance is acidic, blue litmus paper turns red. If the substance is basic or alkaline, red litmus paper becomes blue.
In acidic solutions, litmus turns red, and in basic solutions, it turns blue.
What Is Litmus Paper?
Litmus Paper is a type of paper that changes color in reaction to the acidity of the solution in which it is dipped and can thus be used to assess acidity. It is a filter paper that has been coated with a natural-soluble color derived from lichen.
Litmus paper is a type of paper that generates a result that may be used to determine the pH of a solution. It is frequently absorbed into filter paper to make one of the oldest kinds of pH indicator, which is used to assess the acidity of materials. Blue litmus paper turns red in an acidic condition, while blue litmus paper turns blue in a basic or alkaline medium.
The color change happens over a pH range of 4.5-8.3 at 25 degrees Celsius (77 degrees Fahrenheit).
Under acidic conditions, light blue litmus paper turns red, while red litmus paper turns blue under basic or alkaline conditions. The neutral indication is purple litmus paper.
History
Arnaldus de Villa Nova, a Spanish alchemist, used litmus for the first time around 1300 CE. Since the 16th century, blue dye has been extracted from lichens. The term “litmus” is derived from the Norse word for “dye” or “color.”
While all litmus paper behaves similarly to pH paper, the contrary is not true. All pH paper should not be referred to as “litmus paper.”
Sources
Litmus is mostly obtained from the species Roccella montagnei and Dendrographa leucophoea. Roccella tinctoria, Roccella fuciformis, Roccella phycopsis, Rocella pygmaea, Ochrolechia parella, Parmotrema tinctorum, Variolaria dealbata, and Parmelia sp. are among the other species that produce the pigment. The lichens contain anywhere from 10 to 15 indicator chemicals. Some of these, such as azolitmin and erythrotmin, are separable and act on their own.
Colors of Litmus Paper
Litmus paper comes in three colors: purple, blue, and red. The dyes used in both blue and red litmus paper are present in purple litmus paper.
Purple Litmus Paper: Purple litmus paper remains purple in neutral pH solutions, turns red in acidic pH solutions, and turns blue in basic pH solutions.
Blue Litmus Paper: Blue litmus paper remains unchanging blue or sometimes purple in neutral pH solutions, turns red in acidic pH solutions, and dark blue in solutions with pH greater than 8.3.
Red Litmus Paper: Red litmus paper remains red or sometimes purple in solutions with neutral pH, blue in solutions with basic pH, and dark red in solutions with pH less than 4.5.
When dry, red litmus paper appears more coral-colored than red. When wet, it turns a darker red. When wet, blue and purple litmus paper appear slightly darker, but the difference is minor. At neutral pH, red and blue litmus paper appear unchanged, but they occasionally lean purple. Red and blue litmus paper may not always indicate a neutral pH.
Experiment with Litmus Paper
It is feasible to tell whether a solution is basic or acidic using red and blue test strips or litmus paper, but it is impossible to tell how strong the solution is.
Each number on the pH scale is represented by a different color.
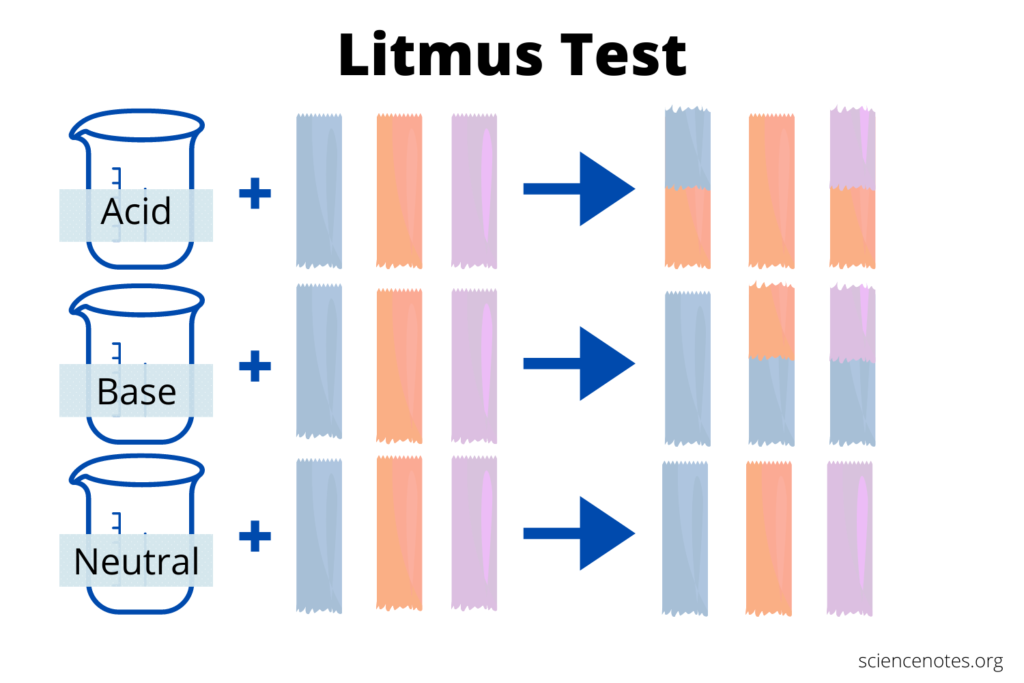
The paper turns green when neutral solutions are employed. Litmus paper is used to determine if a solution is acidic or basic. The litmus paper’s acid or base power is not accurately represented on the paper. Litmus paper is a form of filter paper that has been treated with lichen-derived natural water-soluble pigment.
Procedure:
- Litmus test must be immersed in one of the substances to be tested for contamination. Perform the method a second time using a little piece of blue litmus paper.
- The material tested is acidic if the blue Litmus paper does not change color and the red Litmus paper does not change color.
- This signifies that the material is alkaline if the red Litmus paper turns blue but the blue Litmus paper does not.
Because acids and bases only apply to aqueous (water-based) solutions, pH paper will not change color in non-aqueous liquids like vegetable oil.
To modify the color of a gaseous sample, dampen litmus paper with distilled water. Because the entire surface is exposed, gases alter the color of the litmus strip. The pH paper’s color is unaffected by neutral gases such as oxygen and nitrogen.
Tests for Acids with Litmus Paper
Litmus paper is a natural indication that can be used to assess whether an acidic solution is present. The following actions have to be taken to test the acids with litmus paper:
Step 1: Place the unidentified solution in the beaker.
Step 2: Cut a strip of purple or blue litmus paper, whichever you want.
Step 3: Dip the tip of the purple or blue litmus paper into the appropriate solution.
When the blue litmus paper turns red, the given solution is acidic.
Tests for Bases with Litmus Paper
Litmus paper is a natural indicator that can be used to determine whether or not a particular answer is fundamental. The following actions have to be taken in order to test the bases with litmus paper:
Step 1: Put the unidentified solution in the beaker.
Step 2: Cut a strip of purple or red litmus paper, whichever you want.
Step 3: Dip the tip of the litmus paper into the appropriate solution.
If the purple or red litmus paper turns blue, the stated solution is simple.
Litmus Paper Use in Chemistry Lab
Litmus paper is a low-cost, portable chemical lab device for testing acidity and alkalinity using only a small volume of solution. The pH of the compounds must be determined.
Other chemical processes beyond acid-base can induce a color shift in litmus paper. For example, chlorine gas turns blue litmus paper white; the litmus dye is bleached due to the presence of hypochlorite ions. Because this reaction is irreversible, the litmus does not serve as an indicator in this situation.
The pH of the material must be tested since most living forms can only live in a narrow pH range, making pH a significant biological indicator. One of the delicate balances is the acid-base balance in the human body. Even a minor change in the pH value of the blood in the body might result in death. Little pH changes in the soil effect not just humans and animals, but also plants.
| Litmus Paper | pH value | color indications |
| Purple Litmus Paper | Neutral pH value below 4.5 pH value above pH 8.3 | No change Red Blue |
| Blue Litmus Paper | Neutral pH value below 4.5 pH value above pH 8.3 | No change Slight Purple Red Blue |
| Red Litmus Paper | Neutral pH value below 4.5 pH value above pH 8.3 | No change Red Blue |
Limitations
This is why too-acidic soil is tempered with calcium carbonate.
The litmus test is quick and easy to use, but it has a few drawbacks. Secondly, it is not an exact pH indicator; it does not produce a numerical pH value. Instead, it tells whether a sample is acidic or basic. Second, the paper’s color can vary for causes other than an acid-base reaction.
Blue litmus paper, for example, turns white when exposed to chlorine gas. This color change is caused by hypochlorite ion bleaching, not acidity/basicity. A base is a fertilizer.
References
- Beecken, H.; E-M. Gottschalk; U. v Gizycki; H. Krämer; D. Maassen; H-G. Matthies; H. Musso; C. Rathjen; Ul. Zdhorszky (2003). “Orcein and Litmus”. Biotechnic & Histochemistry. 78 (6): 289–302. doi:10.1080/10520290410001671362
- Musso, H.; Rathjen, C. (1959). “Orcein dyes. X. Light absorption and chromophore of litmus”. Chem. Ber. 92 (3): 751–3. doi:10.1002/cber.19590920331
- https://unacademy.com/content/ neet-ug/study-material/chemistry/litmus-paper/
- https://tutormate.in/ cbse-class-10-chemistry/indicators-for-testing-acids-and-bases/’
- https://www. scienceequip.com.au/ blogs/news/litmus-paper-everything- you-need-to-know
- https://www.thoughtco.com/what-is-litmus- paper-3976018
- https://byjus.com/ chemistry/litmus-paper/
- Beecken, H.; E-M. Gottschalk; U. v Gizycki; H. Krämer; D. Maassen; H-G. Matthies; H. Musso; C. Rathjen; Ul. Zdhorszky (2003). “Orcein and Litmus”. Biotechnic & Histochemistry
- https://www.toppr.com/guides/chemistry/solutions/litmus-paper-and-litmus-test/#Litmus_Paper
