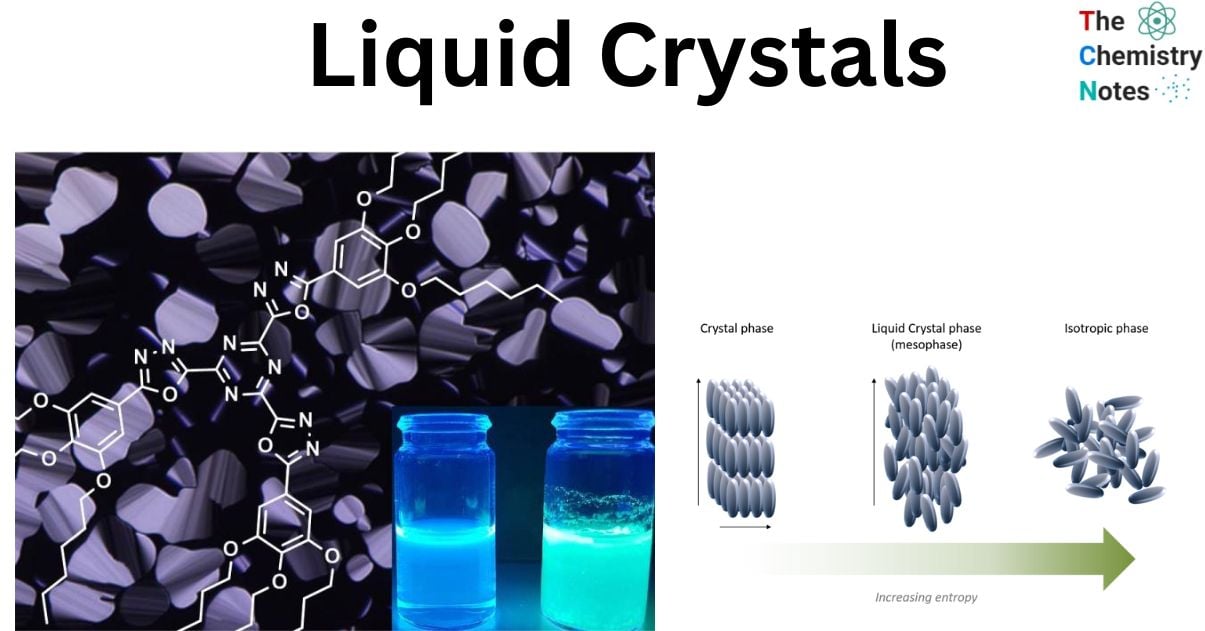
Liquid crystals (LCs) are a kind of matter that exists between crystalline solids, which have a regular periodic arrangement of atoms or molecules, and isotropic liquids, which have no order.
The study of liquid crystals began in 1888, when an Austrian botanist called Friedrich Reinitzer discovered that cholesteryl benzoate had two unique melting points. Reinitzer increased the temperature of a solid sample in his studies and observed the crystal transform into a murky liquid. As he raised the temperature even higher, the substance transformed back into a clear, translucent liquid. Reinitzer is frequently credited with discovering a new phase of matter – the liquid crystal phase – as a result of his early study.
What is Liquid Crystal?
Liquid crystals, a particular type of state of matter referred as the fourth state of matter, or mesophase, is a separate intermediate phase that some compounds create between the solid (crystalline) and liquid (isotropic) phases.
These substances exhibit both solid and liquid-like characteristics.
Given that it typically occurs in the temperature range between the solid and isotropic liquid phases and is characterized by anisotropy of characteristics without a three-dimensional crystal lattice, a liquid crystal is a thermodynamically stable phase.
The molecules, or mesogens, in a liquid crystalline material have anisotropic intermolecular interactions, which means that they have some orientational or positional order but with a lower degree of organization than in a crystalline solid. This indicates that liquid crystal has a flowing behavior akin to a liquid, but because to their positional arrangement, such compounds are frequently more viscous. It is believed that liquid crystals are sensitive to a variety of stimuli, including temperature, electric and magnetic fields. This sensitivity, together with liquid crystals’ propensity for self-assembly, is what makes them so fascinating.
Characteristics of Liquid Crystals
- The majority of liquid crystal phases have a hazy appearance, which means that they scatter light similarly to milk-like colloids. Small groups of molecules form and disperse, leading to fluctuating zones of non-uniformity that cause this light scattering.
- Liquid crystals display birefringence as a result of their anisotropy. Hence, light that enters the crystal is split into two oppositely polarized photons that move at various speeds. A birefringent substance can exhibit stunning patterns and color effects when seen between crossed polarizing filters.
- The unusual ray has a little longer passage through the material than the ordinary ray, thus it emerges later (and out of phase) with the former, which causes interference that results in the hues.
- Like all other types of matter, liquid crystals are susceptible to heat expansion. When a proper liquid crystal combination is painted on a patient’s body, it can frequently identify the locations of tumors or infections because they alter local blood flow and cause temperature anomalies. By printing a series of appropriately prepared LC mixes on a paper or plastic strip that is kept in contact with the surface whose temperature is to be monitored, inexpensive thermometers can be created.
- In the majority of liquid crystal compounds, polymorphism—a circumstance in which more than one phase is seen in the liquid crystalline state—occurs. The “subphases” of liquid crystal materials are referred to as mesophase. Mesophases are created by altering the sample’s level of order, either by imposing order in only one or two dimensions or by allowing some degree of translational mobility in the molecules.
Types of Liquid Crystals
There are numerous ways to categorize liquid crystals; for example, the molecules that make up the mesophases (mesogens) can be calamitic (rod-like), discotic (disc-like), amphiphilic, non-amphiphilic, low molecular weight, polymeric, or contain metals. Either thermotropic or lyotropic behavior is displayed by liquid crystals. Compounds that exhibit thermotropic behavior fall within a specific temperature range, below which they are crystalline and above which they are isotropic liquids. Lyotropic liquid crystals depend on solvents, and the amount of the solvent has an impact on how the liquid crystals aggregate and behave.
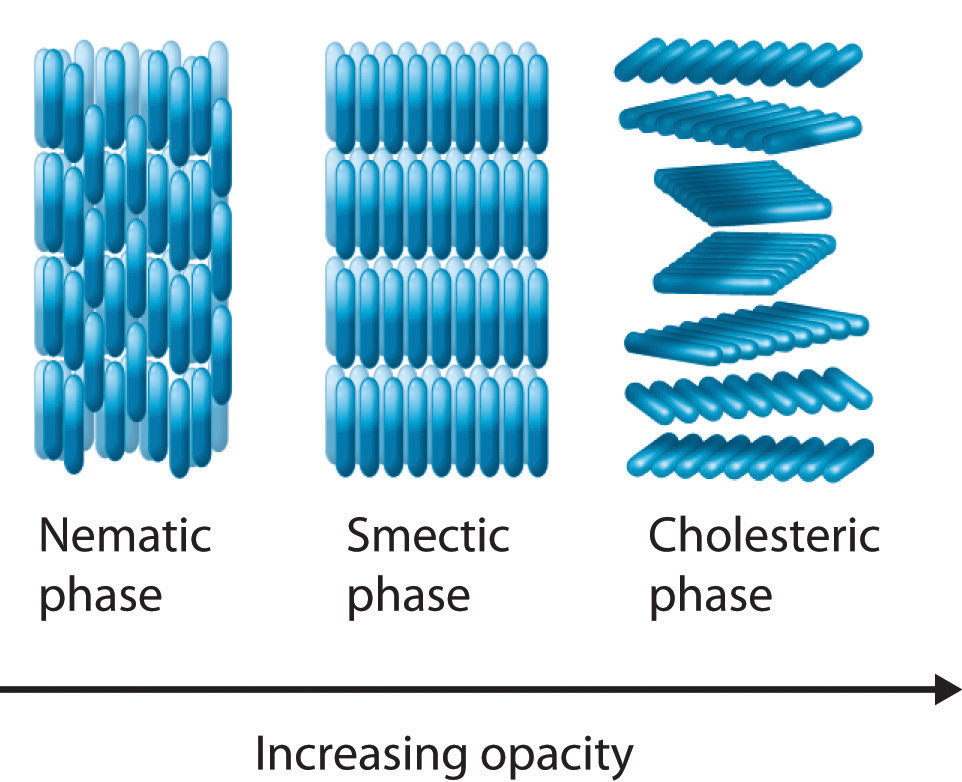
Nematic Phase
The simplest type of liquid crystal is nematic, which is characterized by the crystal molecules’ freedom of movement and lack of organized locations. The molecules do not have a particular arrangement during this phase, but they all point in the same direction, setting them apart from a pure liquid.
The molecules in a nematic phase—the name meaning “thread-like”—are aligned in the same direction yet are allowed to wander around at random, just like they would in a regular liquid. It is possible to manipulate the alignment of the rod-like molecules due to their polarity, which forms the physical foundation for liquid crystal displays and some other electro-optic devices.
When seen under a microscope, the liquid crystal at this stage can be identified by its thread-like appearance. Because it enables a clearer view when scientists are confronted with atmospheric turbulence, nematic liquid crystals are frequently used in telescope lenses.
Smectic Phase
The molecules in the smectic phase are arranged in layers while still maintaining the general arrangement of the nematic phase. Depending on the angle established between the molecular axes and the planes of the molecules, the smectic phase can take on a number of different forms. The so-called smectic A phase, which allows molecules to spin around their long axes within a specific plane but prevents them from easily sliding past one another, is the most basic form of this structure.
The molecules are organized in layers in smectic (“soap-like”) phases, with the long molecular axes roughly perpendicular to the laminar planes. Individual layers can slide over one another (thus the “soap-like” character) in a way similar to that observed in graphite since this axis is the only long-range axis of order. There is a certain amount of short-range order within a layer. Smectic and nematic liquid crystals are both used in the creation of liquid crystal display (LCD) displays because it has been discovered that they have quick electro-optical response times.
Cholesteric Phase
The molecules are directionally aligned and stacked in a helical form during the cholesteric phase, with each layer rotated at a little angle to the layers above and below it. However, direct comparisons are somewhat challenging because most compounds only form one of these liquid crystal phases when the solid is melted or the liquid is chilled. As the degree of molecular organization increases from the nematic phase to the cholesteric phase, the liquid becomes more opaque.
A substance cannot become crystalline or solid because of the cholesteric phase, also known as the chiral nematic phase, which is characterized by molecules aligned and stacked in very thin layers at a tiny angle to one another. Another property of this kind of liquid crystal is its propensity to change color when exposed to various temperatures. Thermometers and mood rings are two examples of everyday household goods that use cholesteric liquid crystals.
Applications of Liquid Crystals
Many fields of science, engineering, and device technology have been significantly impacted by liquid crystal technology. Applications for this unique type of material are continually being developed, and they continue to offer efficient answers to a wide range of issues. Some of its important applications are mentioned below:
- The scientific and industrial communities have been particularly interested in the research on the optical and electrical properties of these special substances. Later, research at a variety of businesses, academic institutions, and government laboratories started to concentrate on the uses of these liquid crystals that benefited from their electro-magneto-optic and photoelectric features.
- Liquid Crystal Displays
Liquid crystal displays (LCDs) are the liquid crystal technology’s most popular application. A multibillion dollar business has developed around this subject, and important advances in science and engineering have been accomplished.
- Liquid Crystal Thermometers
Chiral nematic (cholesteric) liquid crystals reflect light with a wavelength equal to pitch, as was previously shown. The hue of the reflection is influenced by temperature since pitch is temperature-dependent. With the help of liquid crystals, it is able to determine the temperature correctly by observing the thermometer’s color. A device for nearly any temperature range can be created by combining several chemicals.
A few years ago, the “mood ring”—a well-liked novelty—took advantage of the special property of the chiral nematic liquid crystal. Applications that are more significant and useful have been created in a variety of fields, including electronics and medical. To display a “map” of temperatures, special liquid crystal devices can be affixed to the skin. This is helpful since physical issues frequently have a different temperature than the surrounding tissue, such as tumors. By identifying the characteristically higher temperature, liquid crystal temperature sensors can also be utilized to spot faulty connections on a circuit board.
- Optical Imaging
Optical imaging and recording is a use for liquid crystals that is still being researched. A liquid crystal cell is sandwiched between two layers of photoconductor in this technique. The photoconductor receives light, which boosts the material’s conductivity. As a result, a liquid crystal develops an electric field that matches the brightness of the light. An electrode can convey the electric pattern, allowing the image to be recorded. One of the most intriguing fields of liquid crystal research is still under development.
- Cholesteric liquid crystal substances have been used to find veins, arteries, infections, tumors, and the fetal placenta that are warmer than the surrounding tissues when applied to the skin’s surface.
- Nematic liquid crystals are practical research tools for magnetic resonance applications. When molecules are dissolved in nematic liquid crystal solvents, the resulting NMR spectra are extremely well resolved and show intermolecular dipole-dipole fine structures. It is possible to learn about the anisotropy of chemical shifts, direct magnetic dipole-dipole interaction, indirect spin-spin couplings, bond angles, bond lengths, molecular order, and relaxation process by analyzing the spectra of molecules in liquid crystal solvents.
- In chromatographic separations, liquid crystals have been employed as solvents to control the course of chemical reactions, to examine molecular configurations and kinetics, and as an anisotropic host fluid for the visible, UV, and IR spectroscopy of organic molecules.
- Liquid crystal makeup removers, lipsticks, and lip glasses with cholesteric liquid crystals are among the many cosmetic products made with liquid crystals.
- The pharmaceutical industry makes considerable use of liquid crystals.
- Calculators, digital watches, oscillographic systems, and television displays that use L.C. screens have all been developed. Cholesteric liquid crystals have also been utilized in the production of novelty goods including toys and décor.
- There has been a lot of interest in liquid crystal polymers for industrial uses.
Due to its superior surface roughness and low coefficient of friction, polyester liquid crystals were created to be fire resistant and are now utilized as coatings for optical cables and multifibre fabrics. Polyesters with a better elastic modulus are utilized for molding. Mesomorphic free radicals, ferroelectric liquid crystals, and colorless big pitch cholesterol have all been created.
References
- Shao Y, Zerda TW (1998). “Phase Transitions of Liquid Crystal PAA in Confined Geometries”. Journal of Physical Chemistry B. 102 (18): 3387–3394. doi:10.1021/jp9734437.
- Lei L (1987). “Bowlic Liquid Crystals”. Molecular Crystals and Liquid Crystals. 146: 41–54. doi:10.1080/00268948708071801.
- Chemical Properties of Liquid Crystals”. Case Western Reserve University. Archived from the original on November 25, 2012. Retrieved June 13, 2013.
- Chandrasekhar S (1992). Liquid Crystals (2nd ed.). Cambridge: Cambridge University Press. ISBN 978-0-521-41747-1.
- Gennes PG, Prost J (1993). The Physics of Liquid Crystals. Oxford: Clarendon Press. ISBN 978-0-19-852024-5.
- https://pubs.rsc.org/en/content/articlelanding/2023/tc/d2tc04869h/unauth
- Dierking I (2003). Textures of Liquid Crystals. Weinheim: Wiley-VCH. ISBN 978-3-527-30725-8.
- https://www.sciencedirect.com/topics/chemistry/liquid-crystal

Dear madam
Very interesting writing on cholestryl bezoate
I m searching how to synthesis liquid crystal chemical
QC Manager
Oil&Fats