The chromatographic method known as liquid chromatography (LC) is used to separate and analyze mixtures of chemical components in solution to ascertain whether or not a particular component is present. It can be done in either a column or a plane. LC is particularly useful for separating ions or molecules that have been dissolved in a solvent. Since the samples being analyzed do not need to be vaporized, liquid chromatography is more popular than other techniques. In contrast to other forms of chromatography, temperature changes also barely affect liquid chromatography.
Interesting Science Videos
What is Liquid Chromatography?
Liquid chromatography (LC) is a chromatographic technique used to separate ions or molecules that have been dissolved in a solvent. LC is a technique for developing a mixture that has been injected into a column that has been prepared with a suitable stationary phase by passing a liquid through the column in order to separate the mixture into its components by utilizing the difference in retention capacity against the stationary phase and to determine the components.
This method is used for identification, purity testing, and quantitative determination of a liquid or soluble sample.
A column with a fritted bottom holds a stationary phase in equilibrium with a solvent in simple liquid chromatography. Solids, ionic groups on a resin, liquids on an inert solid support, and porous inert particles are examples of commonly used stationary phases. More solvent is added to the column after the mixture to be separated is loaded on top. Due to variations in the partitioning behavior between the mobile liquid and stationary phases, the various mixture constituents move through the column at various rates. Differential elution rates are critical to the success of liquid chromatography. This is determined by molecule adsorption to the stationary phase and molecule solubility within the mobile phase. These properties are determined by the electrochemical and physical properties of both the mobile and stationary phases, most notably polarity and size.
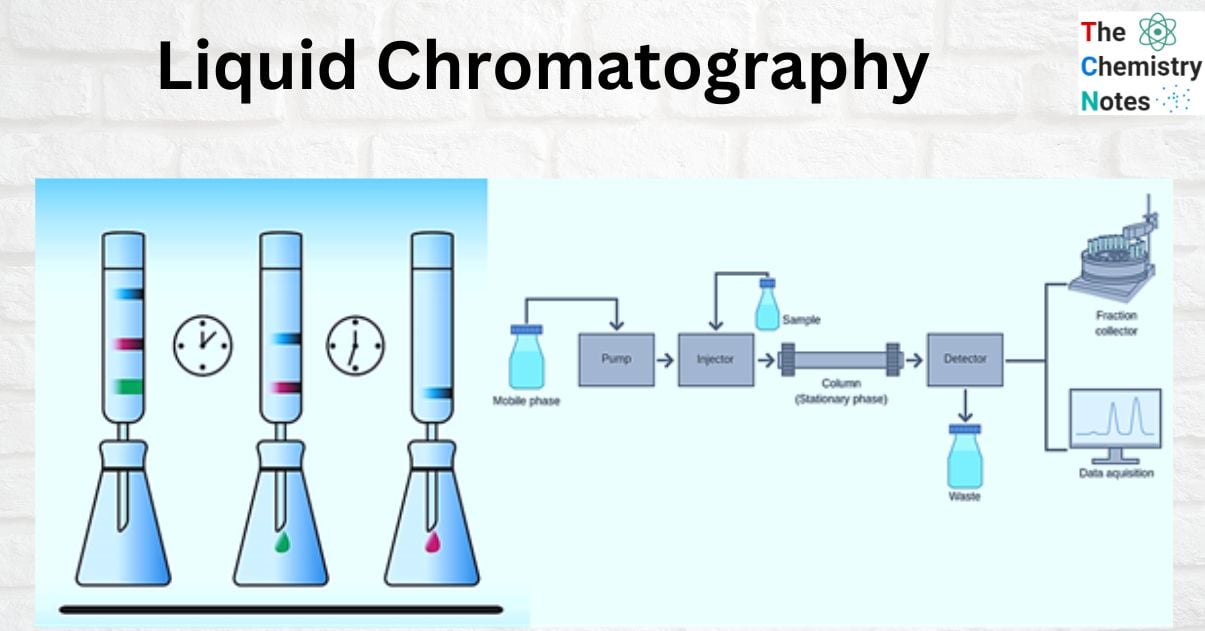
Liquid Chromatography Components
There are numerous types of liquid chromatography (bioaffinity, ion exchange, reverse phase, normal phase, size exclusion, and so on), but they all work on similar basic principles and have similar underlying components.
The most basic instrumentation configurations involve a liquid chromatography column placed beneath a flask or container. When selecting a column, geometric considerations are typically important. Whereas the type of stationary phase used is typically dictated by your liquid chromatography application. Solid silica beads, for example, adsorb molecules in a liquid mobile phase. Whereas ion exchange resins are used in ion-exchange liquid chromatography. As the analyte elutes from the column, it passes through the detector and is analyzed and recorded in a data system for LC.
Procedure of Liquid Chromatography
- A small volume of sample (1-100 µL) is loaded into a sample loop, which is then injected into the mobile phase flow via a six-port valve, triggering the chromatographic run to begin.
- After injecting the sample, the mobile phase is pumped into the column.
- The column is housed in a column oven. As the temperature rises above 45 degrees Celsius, the viscosity of the mobile phase decreases, increasing its linear velocity. This, in turn, reduces run time while also improving chromatographic resolution.
- Components of the mixture with a higher affinity for the mobile phase will migrate quickly through the column with little interaction with the stationary phase. As the component’s band leaves or elutes from the column, the detector will respond in proportion to the component’s concentration.
- The retention time is the time between injection and detection. A component’s retention time will be very specific for a given set of chromatographic conditions and can be compared to that of a standard for identification.
- Less polar analytes will preferentially partition into the non-polar stationary phase and have a longer retention time in reversed-phase chromatography.
- In a chromatogram, the data acquisition system records the detector response as a function of retention time. The chromatogram peaks are usually integrated to calculate the peak area, which is proportional to the concentration of the component present in the sample.
- The separation process is carried out in the column after the autosampler injects the sample into the mobile phase. The chromatographic system’s selectivity has the greatest influence on chromatographic resolution. Changes in the eluotropic strength of the mobile phase (different solvents) or the specific chemical functional groups present in the stationary phase can alter selectivity (changing the column type).
Types of Liquid Chromatography
Partition Chromatography: Both the stationary and mobile phases are liquid in this method. The stationary phase liquid and the mobile phase would be immiscible.
Liquid-Solid Chromatography: The stationary phase has been replaced with a bonded rigid silica or silica-based component onto the inside of the column. Alumina is sometimes used as the stationary phase. This method can be used in both its normal and reverse phases.
Ion Exchange Chromatography: It is a technique used to separate and identify ions on columns with a low ion exchange capacity. This is based on the equilibrium of ion exchange between the ions in solution and the counterions that are fixed to the stationary phase.
Size Exclusion Chromatography: This technique separates molecules based on their size. Smaller molecules will become trapped in the silica particles. They elude from the column at a faster rate than larger molecules. Larger molecules are swept away in the mobile phase, resulting in a shorter retention time.
Affinity Chromatography: In this method of chromatography, the analyte molecules in a sample are bound by a reagent. Typically, the stationary phase is agarose or a porous glass bead capable of immobilizing the bonded molecule. This method is frequently used in biochemistry to purify proteins.
Chiral Chromatography: Chiral chromatography allows liquid chromatography to be used to separate a racemic mixture into its enantiomeric components. A chiral additive can be added to the mobile phase, or a chiral stationary phase can be used. To recognize the chirality of the analyte, the stationary phase must be chiral; this creates attractive forces between the bonds and also forms inclusion complexes.
Applications of Liquid Chromatography
Forensics: Scientists have to be very careful when examining the materials used in explosives. Thus, LC can be used to protect the substance during analysis. For example, by assisting teams in determining what materials were used.
Pharmaceuticals: Liquid chromatography is also used in the pharmaceutical industry. HPLC is used to analyze drug products in order to separate and quantify their various ingredients. This step ensures that drug products are manufactured in a safe and consistent manner.
Illegal drug use: Liquid chromatography is one method of detecting illegal substance use. Liquid chromatography can separate the specific illicit component from a sample if the police want to prove that someone was driving under the influence of drugs or simply took illegal drugs.
Food inspection: Food products, like pharmaceuticals, must have the right ingredients in the right quantities, as well as avoid the wrong ingredients. LC can detect unwanted ingredients in food, such as veterinary drugs and pesticides. It can also be used to separate specific compounds in order to determine what contributes to a particular flavor or aroma profile in a product.
Petrochemicals: Plastics, solvents, fertilizers, paint products, and insulating materials are all part of the petrochemical industry. As a result, products must perform as expected. To accomplish this, manufacturers and product developers must conduct accurate tests.
Chemical Production: Liquid chromatography is used to verify that the right chemical was made after a chemical has been manufactured.
Environmental Contamination: When a harmful substance has contaminated the environment, LC is used to identify the pollutants that are present.
Advantages of Liquid Chromatography
- When all of the necessary parameters and equipment are used, liquid-solid column chromatography is an effective separation technique.
- This method is especially useful when the compounds in the mixture are colored, as it allows the scientist to see the separation of the bands for the components in the sample solution. Even if the bands are not visible, certain components can be seen using other methods of visualization.
- Irradiation with ultraviolet light is one method that may be effective for some compounds. This makes collecting samples one after the other relatively simple.
- Liquid-solid column chromatography is also a less expensive method of separation than other methods (HPLC, GC, etc.). This is due to the fact that the most basic forms of column chromatography do not necessitate the use of expensive machinery such as high pressure solvent pumps used in HPLC.
- Furthermore, the glass wear used in liquid-solid column chromatography is reasonably priced and widely available in many laboratories.
- Burets are commonly used as the separating column, which works just as well as an expensive pre-prepared column in many cases. Pasteur pipettes are frequently used for small-scale chromatography.
Liquid Chromatography Limitations
- If the components of the solution are not visible using any of these methods, determining the efficacy of the separation can be difficult. Separate collections from the column are taken at specified time intervals in this case.
- The flow of the mobile phase, detection of each separation band, and collection of each component are all done manually by the scientist in methods other than flash chromatography. Thus, this may introduces many potential instances of experimental error.
- An error occurs due to sample overloading.
References
- https://www.chromatographytoday.com/news/lcxlc/49/breaking-news/5-applications-of-liquid-chromatography/57957
- https://byjus.com/chemistry/liquid-chromatography/
- https:// www.lcservicesltd.co.uk/everything-you-need-to-know-about-liquid-chromatography/
- https://www.technologynetworks.com/analysis/articles/ liquid-chromatography-including-hplc-uhplc-and-lcxlc-344048
- https://www.bio-rad.com/en-np/applications-technologies/liquid-chromatography-principles?ID=MWHAS7E8Z
- https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Instrumentation_and_Analysis/Chromatography/Liquid_Chromatography
