Definition of Lewis Acids and Bases
- Gilbert Newton Lewis (American, 1923) put forward a broader concept of acids and bases on the basis of electron transfer. This concept is called the Lewis concept.
- Lewis’s concept eliminates the necessity of the presence of hydrogen in acid, and it accepts not only Arrhenius and Bronsted-Lowry concept but also accepts many other substances that could not be explained through the previous concepts.
- According to this concept, acids are species (charged or uncharged) that can accept an electron pair or electron-pair acceptors are Lewis acids.
- Similarly, bases are defined as species that can donate an electron pair, or electron-pair donors are Lewis bases.
- According to this concept, water is also an example of a Lewis base.
- H2O, in this case, donates a pair of electrons so it is a Lewis base and H+ accepts a pair of electrons, so it is a Lewis acid.
Reactions of Lewis Acids and Bases
The following are some reactions between Lewis acids and bases.
1. Molecules having a central atom with an incomplete octet are Lewis acids whereas anions like Cl–, F–, I– having a pair of non-bonding electrons are Lewis base. The underlined central atoms in BF3, AlCl3, FeCl3 molecules have only six electrons, and they show the tendency to accept electrons in order to complete their octet.
Reaction:
Cl– (Lewis base) + AlCl3 (Lewis acid) → [Cl → AlCl3]+
- In this reaction, Cl– has non-bonding pairs of valence electrons, so it is a Lewis base. Al3+, however, has empty 3s, 3p, and 3d orbitals that can be used to hold pairs of electrons donated by other species.
2. Molecules with central atom deficient with electrons (empty or vacant d-orbitals) are Lewis acids. They can extend their valence shell and can accommodate more electrons.
Reaction:
SiF4 (Lewis acid) + 2F– (Lewis base) → [SiF6]2-
SnCl4 (Lewis acid) + 2Cl– (Lewis base) → [SnCl6]2-
3. Simple cations like Ag+, Cu2+, Li+, Fe3+ are Lewis acids as they can accept a pair of electrons while molecules like NH3 having a pair of lone electrons act as a Lewis base.
Reaction:
H3N: + Ag+ + :NH3 → [NH3 → Ag → NH3]
Cu2+ + 4(:NH3) → [Cu(NH3)4]2+
4. Molecules having multiple bonds between two atoms of different electronegativities are Lewis acids.
Reaction:
CO2 + H2O → H2CO3
CO2 contains double bonds between carbon and oxygen (O=C=O). Since oxygen is more electronegative than carbon, carbon acquires a slight positive charge and thus can accept a pair of electrons.
Examples of Lewis acids and bases
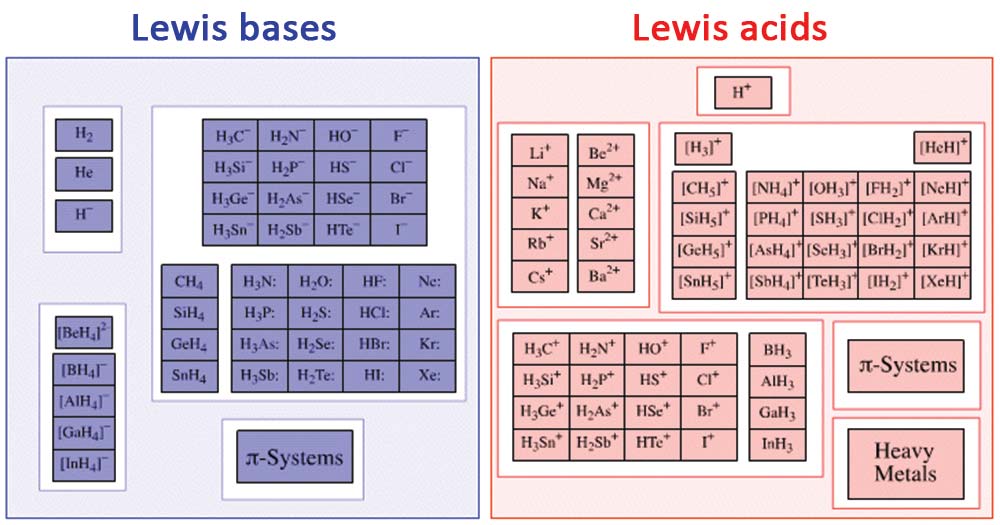
Figure: Diagram of some Lewis bases and acids. Image Source: Evans
Lewis Acids
- AlCl3
- BF3
- FeCl3
- Ag+
- Cu2+
- Fe3+
- CO2
- SO2
- SnF4
- PF5
- SiF4
Lewis Base
- H2O
- NH3
- Cl–
- F–
- I–
- H–
- CH3–
- SbCl5
- SO42-
- C2H2
Applications
- Lewis acids are commonly used as a catalyst in different alkylation reactions.
- One example of this is the use of AlCl3 in Friedel–Crafts alkylation reaction where AlCl3 accepts a pair of electrons to form a strongly acidic and electrophilic carbonium ion
RCl + AlCl3 → R+ (carbonium) + AlCl4−
- Lewis bases are used in fields like pharmacology, where they are used to modify the activity and selectivity of a metal catalyst.
Advantages of Lewis Concept
- This concept includes reactions in which no protons are involved.
- It is more general and extended as compared to other concepts.
- It includes the basic properties of metallic oxides and acidic properties of non-metallic oxides.
- This concept is of great value in cases where the protonic concept is inapplicable.
- It can also explain that H+ and OH- are Lewis acid and Lewis base, respectively.
Limitation of Lewis Concept
- It fails to account for the strength of acids and bases as it does not consider the ionization process. The strength of acids and bases is found to depend on the type of reaction; it is not possible to arrange Lewis acids and bases in any order of their relative strength.
- Acids-bases reactions are instantaneous reaction and occur very rapidly. But there are many Lewis acid-base reactions that are slow. In fact, the formation of a coordinate covalent bond (Lewis concept) is a slow process.
References
- Gautam SD, Pant M, and Adhikari NR (2016). Comprehensive Chemistry, Part 2. Sixth Edition. Heritage Publishers and Distributors Pvt. Ltd.
Internet Sources
- 2% – https://chemed.chem.purdue.edu/genchem/topicreview/bp/ch11/lewis.php
- 1% – https://schoolpk.org/acids-bases-and-salts/
- 1% – https://quizlet.com/24073818/unit-14-acids-and-bases-flash-cards/
- 1% – https://chem.libretexts.org/Bookshelves/General_Chemistry/Book%3A_Chem1_(Lower)/10%3A_Fundamentals_of_Acids_and_Bases/10.05%3A_Lewis_Acids_and_Bases
- 1% – https://brainly.com/question/4205539
- 1% – http://www.wiredchemist.com/chemistry/instructional/general-chemistry-modules/acidsbases/acids-and-bases/lewis
- 1% – http://chemistry.elmhurst.edu/vchembook/185strength.html
- <1% – https://www.youtube.com/watch?v=AZ2CEsPAeHo
