The Le Chatelier’s principle is one of the key ideas in chemical equilibrium. It enables us to forecast how an equilibrium system will behave in diverse situations. Changes in both concentration and temperature affect the position of equilibrium. When any of the reactants or products are gases, changes in pressure may also affect the position of equilibrium. French chemist Henri Le Chatelier (1850–1936) observed how these factors affect the position of equilibrium.
What is Le Chatelier’s principle?
Le Chatelier’s Principle states that when a chemical system is under stress and in equilibrium, the equilibrium will change to lessen the stress. In response to a change in temperature, concentration, volume, or pressure, it can be used to forecast the course of a chemical reaction. Le Chatelier’s principle can be used to predict how a system will react to a change in equilibrium, but it cannot (at the molecular level) explain why the system will react the way it does.
French chemist Henri Le Chatelier put forward a general rule, known as Le Chatelier’s principle:
If one or more factors that affect an equilibrium is changed, the position of equilibrium shifts in the direction that reduces (opposes) the change.
We can predict the effect of changing concentration and pressure by referring to the stoichiometric equation for the reaction. We can predict the effect of changing the temperature by referring to the enthalpy change of the reaction.
Effect of concentration in the position of equilibrium
When the concentration of one or more of the reactants is increased:
- the system is no longer in equilibrium
- the position of equilibrium moves to the right to reduce the effect of the increase in concentration of reactant
- more products are formed until equilibrium is restored. When the concentration of one or more of the products is increased:
- the system is no longer in equilibrium
- the position of equilibrium moves to the left to reduce the effect of the increase in concentration of product
- more reactants are formed until equilibrium is restored
For example, look at the reaction:
CH3COOH (l) + C2H5OH (l) ⇌ CH3COOC2H5 (l) + H2O (l)
ethanoic acid ethanol ethyl ethanoate water
Condition 1: What happens when we add more ethanol?
- The concentration of ethanol is increased.
- According to Le Chatelier’s principle, some of the ethanol must be removed to reduce the concentration of the added ethanol.
- The position of equilibrium shifts to the right.
- More ethanol reacts with ethanoic acid and more ethyl ethanoate and water are formed
In Condition 2: What happens when we add more water?
- The concentration of water is increased.
- According to Le Chatelier’s principle, some of the water must be removed to reduce the concentration of the added water.
- The position of equilibrium shifts to the left.
- So, more water reacts with ethyl ethanoate and more ethanoic acid and ethanol are formed
Condition 3: What happens when we remove some water?
- The concentration of water is decreased.
- According to Le Chatelier’s principle, some water must be added to increase its concentration.
- The position of equilibrium shifts to the right.
- So more ethanoic acid reacts with ethanol and more water and ethyl ethanoate are formed
Effect of pressure on the position of equilibrium
Change in pressure only affects reactions where gases are reactants or products. The molecules or ions in solids and liquids are packed closely together and cannot be compressed very easily. In gases, the molecules are far apart.
The pressure of a gas is caused by the molecules hitting the walls of the container. Each molecule in a mixture of gases contributes toward the total pressure. So, at a constant temperature, the more gas molecules there are in a given volume, the higher the pressure
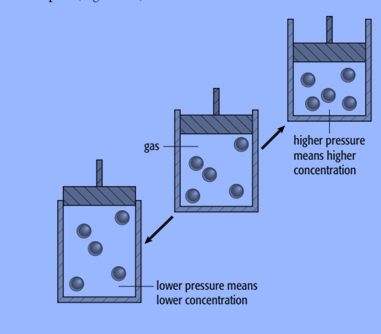
For example, consider the reaction: 2SO2 (g) + O2 (g) ⇌ 2SO3 (g)
There are three moles of gas molecules on the left of the equation and two on the right.
Condition 1: What happens when we increase the pressure?
- The molecules are closer together because the pressure is higher.
- According to Le Chatelier’s principle, the reaction must shift in a direction that reduces the number of molecules of gas.
- The position of equilibrium shifts to the right.
- So, more SO2 reacts with O2 to form SO3.
Condition 2: What happens when we decrease the pressure?
- The molecules are further apart because the pressure is lower.
- According to Le Chatelier’s principle, the reaction must shift in a direction that increases the number of molecules of gas.
- The position of equilibrium shifts to the left.
- So, more SO2 and O2 molecules are formed by the decomposition of SO3 molecules.
The reaction moves toward the side with lower pressure as the pressure or volume increases. Equilibrium moves toward the higher pressure side of the equation if either the pressure or volume is increased.
Effect of Change of Temperature on Equilibrium
Each reaction within the equilibrium can either be exothermic or endothermic. Similar to this, the reversible reactions may be endothermic or exothermic at equilibrium depending on the amount of net energy involved.
Le Chatelier’s Principles state that- When temperatures are in exothermic equilibrium, rising temperatures cause product creation to decrease and falling temperatures to enhance it. In endothermic processes, the rising temperature has a positive impact on product formation while decreasing temperature has a negative one.
An endothermic reaction takes place when hydrogen iodide breaks down.
2HI ⇌ H2 + I2 ΔHr = +9.6 KJ mol–1
The concentration of the product rises as the temperature rises. The position of equilibrium shifts to the right.
We can explain this using Le Chatelier’s principle:
- an increase in temperature increases the energy of the surroundings
- according to Le Chatelier’s principle, the reaction will go in the direction that opposes the increase in energy
- and hence the reaction will go in the direction in which energy is absorbed, which is the endothermic reaction
- position of equilibrium shifts to the right, producing more H2 and I2.
If a temperature increase favors an endothermic reaction, a temperature reduction must favor an exothermic reaction:
- a decrease in temperature decreases the energy of the surroundings
- Le Chatelier’s principle states that the reaction will go in the direction that opposes the decrease in energy
- Hence, the reaction will go in the direction in which energy is released, which is the exothermic reaction.
Impact of Catalyst on the equilibrium position
A catalyst is something that speeds up a chemical reaction. Catalysts shorten the period of time needed to reach equilibrium, but they have no impact on the equilibrium’s location once it has been attained. This is due to the fact that they equally speed up forward and reverse reflexes.
Applications of the Le Chatelier’s Principle
According to Le Chatelier’s principle, parameters like concentration, pressure, and temperature have an impact on where an equilibrium lies in a reversible reaction. This can help us raise a reaction’s yield.
Le Chatelier’s approach is used in numerous industrial processes to boost yield but frequently under unfavorable circumstances. These strike a compromise between high yield and reaction rate and cost.
Le Chatelier’s principle is used in the preparation of methanol, ethanol, sulphuric acid, and ammonia, among other reversible processes.
Read More: Energy Diagram
References
- Atkins, P.W. (1993). The Elements of Physical Chemistry (3rd ed.). Oxford University Press.
- https://www.studysmarter.co.uk/explanations/chemistry/physical-chemistry/application-of-le-chateliers-principle/
- https://byjus.com/jee/le-chateliers-principle-on-equilibrium/
- https://chemistrytalk.org/le-chateliers-principle/
- https://Cambridge-International-AS-A-Level-Chemistry- 9701/
- https://www.thoughtco.com/definition-of-le-chateliers-principle-605297
