Who is Joseph Proust?
- Joseph Louis Proust (26 September 1754- 5 July 1826) was a French chemist who is best known for the discovery of the law of definite proportions or the law of constant composition.
- Joseph Proust was a chemistry teacher who taught in different schools like the Royal Artillery School in Segovia and Musee, which is a private teaching institution.
- Proust founded the law of definite proportions (also known as Proust law) in 1794, during his work on copper carbonate, two tin oxides, and iron sulfides.
Law of definite proportions definition
The law of definite proportions states that a chemically pure substance always contains the same set of elements combined together in a definite proportion by weight.
- In other words, the law can be stated as the percentage composition of an element in a compound is always fixed.
- The law of definite proportions is crucial in analytical chemistry and forms the basis of various other chemical discoveries.
- The law explains that the chemical composition of a chemically pure substance is the same irrespective of the source and the elements in the compound exist in the same ratio by mass.
- There has been a correlation of the law of definite proportions with Dalton’s atomic theory which was later established by Swedish chemist Jacob Berzelius in 1811.
- The law is also in accordance with the law of conservation of mass by Lavoisier and Lomonosov.
Read Also: Law of conservation of mass- definition, formula, equation, examples
Law of definite proportions formula
The proportion of different elements in a compound is obtained by the following formula;
If a compound AB is composed of two elements; A and B, the proportions/ percentage ratios of the elements can be expressed as;
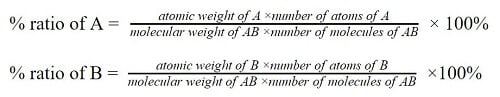
Law of definite proportions equation
The law of definite proportions can be explained by the following mathematical expression;
If a compound AB is obtained from three different sources with the following composition;
x1 gram of A and y1 gram of B
x2 gram of A and y2 gram of B
x3 gram of A and y3 gram of B
Now, by the law of definite proportions;
x1/y1 = x2/y2 = x3/y3
This indicates that the percentage composition of the element A and B is fixed in the compounds obtained from three different sources.

Law of definite proportions examples
1. Hydrogen, Oxygen, and Carbon in Glucose
- Glucose is a molecule with a molecular formula C6H12O6, indicating that glucose is composed of carbon, hydrogen, and oxygen in different proportions.
- For a compound to be glucose or for a molecule of glucose to form, 6 atoms of carbon, 12 atoms of hydrogen, and 6 atoms of oxygen are required.
- In terms of percentages, carbon makes up about 40% of glucose, hydrogen makes up 7%, and oxygen makes up 53% of a glucose molecule.
- A glucose molecule cannot be formed if any of the above proportions are changed. Similarly, glucose obtained from different sources, natural or artificial, comprises the same elements in the same proportions as the compound follows the law of definite proportions.
2. Hydrogen and oxygen in the water
- Water is composed of two atoms of hydrogen atoms combined with a single atom of oxygen by covalent or hydrogen bonding.
- The molecular weight of one mole of water is 18 grams which contains 2 grams of hydrogen and 16 grams of oxygen.
- In terms of percentage by weight, hydrogen makes up 11% of water whereas oxygen makes up 89% of a water molecule.
- The ratio of the elements in water remains the same even if the weight or moles of water are increased.
- Water present in any state of matter contains the elements in the same proportion as the law of definite proportion holds true for water.
Exceptions to the Law of Definite Proportions
Even though the law of definite proportions is a building block of modern analytical chemistry, the law doesn’t hold true for all chemical compounds. Some of the exceptions to this law are;
- The law of definite proportions holds true for all molecules in the gaseous state and for smaller molecular compounds in other states of matter, but it is not valid for polymers. The polymers with even identical monomers have considerable variation in the polymer molecule length, branching, termination, etc.
- The law is not followed by non-stoichiometric compounds with varying compositions of elements when obtained from different sources. Nonstoichiometry is commonly observed in compounds of the transition and inner transition elements as they have varying oxidation states and multiple oxidation states of the elements can occur together in a solid phase.
- The isotopic composition of the constituent elements varies in various samples of a compound which causes deviation from the law. This is due to fluctuations in the mass ratios of the elements.
References and Sources
- Gautum SD, Pant M and Adhikari NR (2016). Comprehensive Chemistry, Part 2. Sixth Edition. Heritage Publishers and Distributors, Pvt. Ltd.
- Lawrence Suchow. The failings of the law of definite proportion. Journal of Chemical Education 1975 52 (6), 367. DOI: 10.1021/ed052p367
- https://byjus.com/chemistry/law-constant-proportion/
- 4% – https://en.wikipedia.org/wiki/Joseph_Proust
- 2% – https://quizlet.com/553179267/chemistry-semester-final-flash-cards/
- 2% – https://chemistrygod.com/law-of-definite-proportions/
- 1% – https://www.quora.com/What-is-the-weight-in-grams-of-1-mole-of-water
- 1% – https://www.enotes.com/homework-help/any-help-greatly-appericated-law-definite-327086
- 1% – https://www.britannica.com/science/water
- 1% – https://www.answers.com/Q/What_is_the_ratio_of_carbon_to_hydrogen_to_oxygen_in_glucose
- 1% – https://quizlet.com/94372268/chem-chapter-3-flash-cards/
- 1% – https://quizlet.com/13516986/ap-chemistry-chapter-2-vocabulary-flash-cards/
- 1% – https://hktukaram.blogspot.com/2019/12/
- 1% – https://en.wikipedia.org/wiki/Law_of_definite_proportions
- 1% – https://ciaaw.org/pubs/TICE-1997.pdf
- 1% – https://chem.libretexts.org/Bookshelves/General_Chemistry/Book%3A_Chemistry_(OpenSTAX)/19%3A_Transition_Metals_and_Coordination_Chemistry/19.1%3A_Properties_of_Transition_Metals_and_Their_Compounds
- 1% – https://byjus.com/chemistry/law-constant-proportion/
- 1% – https://brainly.com/question/1507292
- 1% – https://ankplanet.weebly.com/law-of-constant-composition.html
- <1% – https://link.springer.com/chapter/10.1007%2F978-94-017-2292-6_6

wow ..nice teaching thnks..i really understood it now.. nd i appreciate