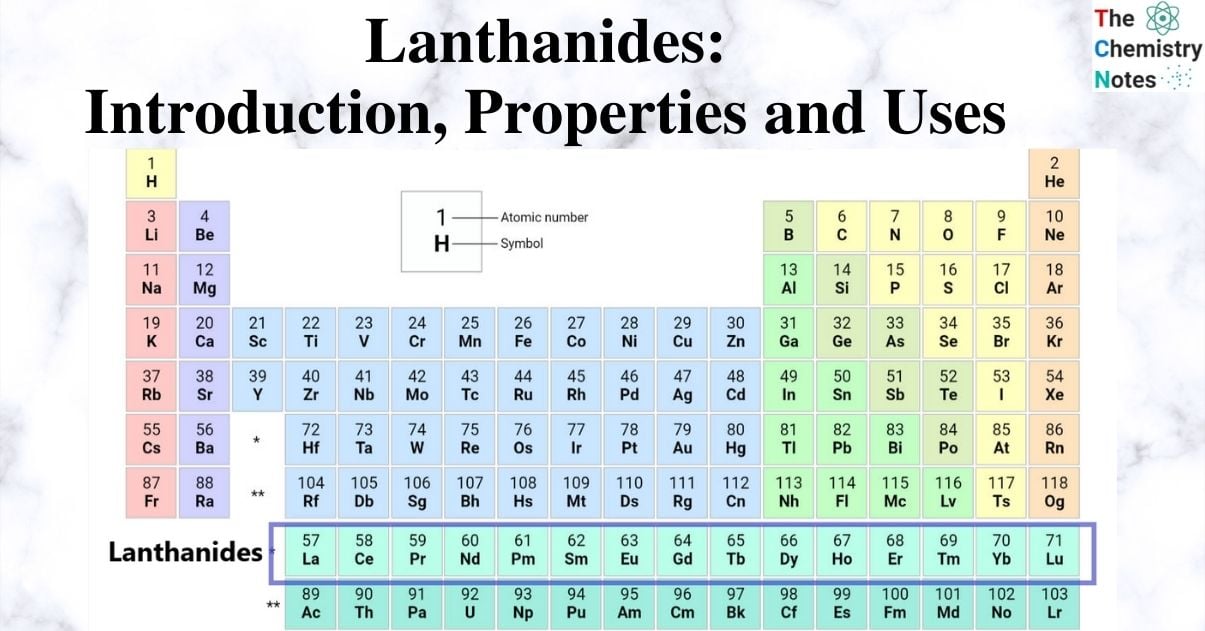
Lanthanides are a set of 15 chemical elements whose atomic numbers range from 57 to 71. These are the elements in the periodic table’s f-block of period six. Each of these elements has one valence electron in the 5d shell. The elements have characteristics in common with lanthanum, the group’s initial element.
Despite being occasionally referred to as rare earth, lanthanides are not very rare. They are hard to differentiate from one another, though. Even though these metals can be categorized as transition metals, they have characteristics that make them distinct from the other elements.
Introduction
The first member of the lanthanides, lanthanum, has an atomic number of 57 and is a real member of Group III. The remaining fifteen elements are those that come after lanthanum, such as those with atomic numbers 58 to 71, where the 4f electrons are successively added to the La configuration. La is included in the name lanthanide because it serves as a precursor for the next 14 elements.
In 1787, a peculiar black mineral was identified in Ytterby, Sweden, leading to the initial discovery of Lanthanides. Professor Gadolin extracted yttria from the mineral in 1794; yttria is an impure form of yttrium oxide. The first Cerium compound was discovered in 1803, by Berzelius and Klaproth. Later, Moseley demonstrated that there were fourteen elements between lanthanum and hafnium using the elements’ x-ray spectra. Later, the same mineral’s remaining constituents were separated. The term Lanthanides was coined after the first element in the series, Lanthanum.
Occurrence
Contrary to what the label “rare earth” might imply, lanthanides are not uncommon in the crust of the planet.
Since all lanthanides are found in minerals with the exception of promethium (which is extremely unstable and not found in minerals), which is an unstable radioactive element, their potential sources are limitless.
Monazite
It includes various light lanthanides (La57 to Eu63). It has 50–70% light lanthanides in it.
- Thorium oxide, ThO = 3-10%
- Silicon oxide, SiO2 = 1-2%
- Phosphorous oxide, P2O5 = 22-30%
- Zirconium oxide, ZrO
- Titanium oxide, TiO2
- Uranium traces
Xenotime
It is a combination of heavier lanthanide phosphates (Gd64 to Lu71). Heavy lanthanides make up 54–65% of it.
- Thorium oxide, ThO2 = 3%
- Uranium oxide, U2O8 = 3.5%
- Zirconium oxide, ZrO2 = 2.3%
Euxenite
Both light and heavy lanthanides are present in this mineral in almost equal amounts. It also contains lanthanide phosphates.
Lanthanides: Elements Name and Symbol
| Element | Symbol | Atomic number |
| Lanthanum | La | 57 |
| Cerium | Ce | 58 |
| Praseodymium | Pr | 59 |
| Neodymium | Nd | 60 |
| Promethium | Pm | 61 |
| Samarium | Sm | 62 |
| Europium | Eu | 63 |
| Gadolinium | Gd | 64 |
| Terbium | Tb | 65 |
| Dysprosium | Dy | 66 |
| Holmium | Ho | 67 |
| Erbium | Er | 68 |
| Thulium | Tm | 69 |
| Ytterbium | Yb | 70 |
| Lutetium | Lu | 71 |
Position in Periodic Table
The 4f series (Lanthanods series) and 5f series are two series that are located at the bottom of the periodic table (Actanoids series). Inner transition elements are the combined 4f and 5f series.
The fifteen Ln elements that fall between Ba (atomic number 56) and Hf (atomic number 72) must be positioned between these two elements.
Barium is similar in quality to Calcium and Strontium and shares its outer electrical structure. So, Barium must come after Strontium (Group II). In a similar manner, Hafnium must come before Zirconium (Group IV). This only leaves one space in Group III below Yttrium for Ln. All fifteen components of Ln must be grouped together because they are quite similar to one another. Yttrium and Ln elements are similar in a number of ways.
- As a result of Ln contraction, Y3+ and Er3+ have the same ionic radius (Y3+ = 0.93 and 0.96, respectively).
- Yttrium is found in nature in the forms of Ytterbite, Xenotime, and other materials that are also the ores of heavier lanthanides.
As a result, all of the Lanthanide elements should fit together. To do this, La is positioned beneath Y, and the remaining fourteen Ln have been individually positioned in the f block, at the bottom of the current periodic table.
General Characteristics of Lanthanides
The chemical and physical characteristics of all the elements in the series closely reflect those of lanthanum and those of one another. The following are some of the important qualities and properties:
- Silvery-white metals develop their oxides when exposed to air and tarnish.
- Soft metals in general. Higher atomic numbers result in a little increase in hardness.
- The radius of each lanthanide 3+ ion falls monotonically across the period (increasing atomic number). This phenomenon is known as “lanthanide contraction.”
- High boiling and melting points.
- Highly reactive
- Hydrogen (H2) will react with water and will be released slowly in the cold or swiftly in the heat. Water and lanthanides frequently interact.
- H2 is produced by reaction with H+ (weak acid) (rapidly at room temperature).
- React with H2 in an exothermic reaction.
- Burn readily in the air.
- They work as effective reducing agents.
- Their chemicals are mostly ionic in nature.
- Many rare earth compounds ignite and burn violently at high temperatures.
- Most rare earth compounds exhibit substantial paramagnetic properties.
- Numerous rare earth compounds exhibit intense UV fluorescence.
- Since these optical transitions are weak, limited, and forbidden, lanthanide ions typically have pale colors.
- The lanthanide and iron ions have opposing magnetic moments.
- The majority of nonmetals and lanthanides easily combine to produce binaries when heated.
- Lanthanide coordination numbers are high (greater than 6; usually 8 or 9 or as high as 12).
Electronic Configuration of Lanthanides
The terminal electronic configuration of lanthanides in the first f-block is [Xe] 4f1-14 5d0-1 6s2. The only synthetically produced radioactive element among the fourteen lanthanides is promethium (Pm), which has atomic number 61. The electrons enter the 4f orbital because the energies of the 4f and 5d electrons are so comparable, leaving the 5d orbital empty.
There are two exceptions: lutetium (Z = 71), where the electron enters the 5d orbital because of the presence of a half-filled d-orbital, and gadolinium, Gd (Z = 64), where the electron enters the 5d orbital because of the existence of a half-filled d-orbital.
| Element | Electronic structure of atoms | Electronic structure of M3+ |
| Lanthanum | [Xe] 5d1 6s2 | [Xe] |
| Cerium | [Xe] 4f1 5d1 6s2 | [Xe] 4f1 |
| Praseodymium | [Xe] 4f3 6s2 | [Xe] 4f2 |
| Neodymium | [Xe] 4f4 6s2 | [Xe] 4f3 |
| Promethium | [Xe] 4f5 6s2 | [Xe] 4f4 |
| Samarium | [Xe] 4f6 6s2 | [Xe] 4f5 |
| Europium | [Xe] 4f7 6s2 | [Xe] 4f6 |
| Gadolinium | [Xe] 4f7 5d1 6s2 | [Xe] 4f7 |
| Terbium | [Xe] 4f9 6s2 | [Xe] 4f8 |
| Dysprosium | [Xe] 4f10 6s2 | [Xe] 4f9 |
| Holmium | [Xe] 4f11 6s2 | [Xe] 4f10 |
| Erbium | [Xe] 4f12 6s2 | [Xe] 4f11 |
| Thulium | [Xe] 4f13 6s2 | [Xe] 4f12 |
| Ytterbium | [Xe] 4f14 6s2 | [Xe] 4f13 |
| Lutetium | [Xe] 4f14 5d16s2 | [Xe] 4f14 |
Oxidation States of Lanthanides
The oxidation state of each member of the lanthanide series is +3.
Samarium, europium, and ytterbium were among the metals previously believed to have +2 oxidation states.
It has been determined via additional research on these metals and their compounds that every lanthanide metal has a +2 oxidation state in its solution complexes.
Some lanthanide metals occasionally display the +4 oxidation state. Due to the metals’ uneven distribution of oxidation state, empty, half-filled, or fully filled f-subshells exhibit high stability. The stability of the f-subshell affects the oxidation state of lanthanides in such a way that cerium is favored in the +4 oxidation state because it develops a noble gas configuration, but it reverts to a +3 oxidation state and behaves as a strong oxidant.
Sm2+, Eu2+, and Yb2+ oxidize in an aqueous solution and lose electrons, which makes them effective reducing agents. On the other hand, Ce4+, Pr4+, and Tb4+ gain electrons and are effective oxidizing agents. Higher oxidation states (+4) of elements are only possible with oxides. Consider the elements Pr, Nd, Tb, and Dy as an example.
The production and stability of every ion in a specific oxidation state can be represented by a relevant Born-Haber cycle of various enthalpy terms such as sublimation, ionization, ion hydration, and so on. The oxidation states of Ln are the result of multiple factors working together.
Chemical Reactivity of Lanthanides
- Although they are more reactive than transition elements, all lanthanides exhibit similarities in their reactivity. This is because the outer 5s, 5p, and 5d orbitals shield unpaired electrons from the inner 4f orbital.
- Except for the exception of CeO2, which combines with hydrogen to create solid hydrides at 300-400 degree Celcius, gets easily tarnished with oxygen and transforms into M2O3 oxides.
- Hydrides get decomposed by water. By heating metal with halogen or the oxide with ammonium halide, halides can be created. While fluorides are insoluble, chlorides are liquescent. While carbonate, phosphate, chromates, and oxalates are insoluble in water, nitrates, acetates, and sulfates are soluble.
Formation of Colored Ions
Transition elements have d subshells that are empty. The electron cloud is affected by the formation of cations by transition elements. There are also some elements whose d subshells are only partially filled, and the energy of all the d orbitals is not constant.
We are aware that when transition elements link with nonmetals, they frequently produce colored compounds.
- Transitional element bonds are not ionic bonds. Because of this, transition elements attempt to polarize anions anytime they are positively charged. The attraction of electron clouds to the cations of transition elements suggests the establishment of a covalent bond. Anion compound polarization will result in coloration.
- Transition metals with incompletely filled d or f subshells that are generating coordination compounds may be colored. Because different ligands split the degenerate orbitals in coordination compounds in different ways, there is a slight energy differential between the various d orbitals. Because the orbital geometries of the axis in subshells d and f are different, energy discrepancies result, and the splitting will vary depending on the ligand.
When light shines on the compounds of transition elements, electrons absorb energy and then excite. These electrons release visible light wavelength when they de-excite. Because of this, transition element compounds have color.
| Elements | Color |
| La3+ | Colorless |
| Ce3+ | Colorless |
| Pr3+ | Green |
| Nd3+ | Lilac |
| Pm3+ | Pink |
| Sm3+ | yellow |
| Eu3+ | Pale pink |
| Gd3+ | colourless |
| Lu3+ | colorless |
| Yb3+ | colorless |
| Tm3+ | Pale green |
| Er3+ | Pink |
| Ho3+ | Pale yellow |
| Dy3+ | Yellow |
| Tb3+ | Pale pink |
Physical Properties of Lanthanides
- Density: Since density is the ratio of a substance’s mass to its volume, d-block elements will have a higher density than s-block components. The density trend in the inner transition series will be the opposite of the atomic radii trend, i.e. density increases with a rising atomic number over time. They range in density from 6.77 to 9.74 g cm-3, which is very high. It rises as the atomic number rises.
- Melting and Boiling Points: Lanthanides have a relatively high melting point, but there is no clear trend in any of these points.
- Materials are categorized according to how they interact with magnetic fields as follows:
- Diamagnetic if repelled
- Paramagnetic if attracted
Due to unpaired electrons in orbitals, the lanthanide atoms and ions other than those of type f0 and f14 are paramagnetic in nature. Lu3+, Yb2+, and Ce4+ are hence diamagnetic.
Both the “spin magnetic moment” and the “orbital magnetic moment” are influenced by unpaired electrons. To calculate the total magnetic moment, the electrons’ spin magnetic moment and orbital angular moment are taken into consideration.
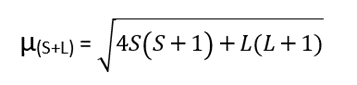
Its unit is Bohr Magneton (BM).
Uses and Application of Lanthanides
Catalytical Application: These substances have the ability to quicken chemical reactions. To remove and stop the accumulation of cooking residues, the interior of the oven’s walls are cleaned using cerium (III) oxide.
Cigarette lighters: Lanthanides are used to create cigarette and gas lighters. The most well-known application of lanthanides was a mixture of cerium and iron that, when struck, produced a bright spark, making it suitable for use as flint in cigarette and gas lighters.
Removal of Sulphur and oxygen impurities: To produce clear, contaminant-free sulfur and oxygen, lanthanides are also employed to remove sulfur and oxygen contaminants from systems.
Oil refinery industries: In the oil refinery industry, lanthanides have been employed to accelerate chemical reactions of various chemicals. They hasten the transformation of crude petroleum into consumer goods like gasoline, which are effective and pure for use right away.
Color television: Europium and yttrium oxides, which contribute to producing red colors on television screens, are responsible for giving colored televisions their color. These substances are lanthanide-related.
Street Lights: On occasion, you might question where the light for a streetlight comes from. Well, searchlights, streetlights, and stadium illumination all make use of lanthanide compounds.
Nuclear applications: Because of their capacity to absorb neutrons, lanthanides are utilized in many different nuclear processes, including rod control, which is used to regulate atomic reactors.
Magnetic Resonance Imaging: Another substance found in the lanthanide elements called gadolinium is employed in hospitals, particularly when utilizing a magnetic resonance imaging scanner to find and diagnose cancerous tumors.
Radioactivity: The radioactively decayed promethium is utilized to create phosphor light, which is subsequently turned into energy by a solar cell, using the resulting factor.
Polymerization: The polymerization procedure uses nitrate and neodymium oxide to accelerate the chemical processes.
References
- J. D. Lee, Concise Inorganic Chemistry, 5th Edition, John Wiley and Sons. Inc. 2007.
- F. A. Cotton, G. Wilkinson & C. Gaus, Basic Inorganic Chemistry, 3 rd Edition, John Wiley & Sons (Asia), Pvt., Ltd., 2007.
- https://en.wikipedia.org/wiki/Lanthanide#Occurrence
- D. F. Shriver & P. W. Atkins, Inorganic Chemistry, 5th Edition, Oxford University Press, 2010.
- https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Descriptive_Chemistry/Elements_Organized_by_Block/4_fBlock_Elements/The_Lanthanides/aLanthanides%3A_Properties_and_Reactions
- https://www.uochemists.com/lanthanides/
- https://pubs.acs.org/doi/10.1021/acscentsci.9b00642
- https://byjus.com/jee/lanthanides/
- https://allusesof.com/chemical/21-uses-of-lanthanides/
- https://sciencenotes.org/lanthanides-facts-lanthanoids/
