To understand the matter, one can employ the kinetic particle theory. And virtually everything around us is made of matter.
All matter is composed of numerous tiny particles that move randomly all the time.
The matter is a substance that has mass and occupies space. Solids, liquids, and gases are the three states of matter that can exist.

Properties of Solids, Liquids, and Gases
Solids
- They are highly dense and have a definite volume and shape.
- Although the atoms vibrate in place, they are unable to move.
- The particles are arranged in a set and regular arrangement, very closely packed together.
Liquid
- In addition to having a set volume, liquids also take on the shape of the container.
- Water is an exception, although they are often denser than gases and less dense than solids.
- Because the particles move and slide past one another, liquids take on the shape of a container and can flow freely.
Gases
- Unlike liquids, which have an arbitrary volume, gases conform to the geometry of the container.
- Gases are exceedingly light in weight.
- A large amount of space between the particles makes it possible to compress gases into a very compact volume.
- The particles are scattered widely, moving randomly at a high speed (about 500 m/s) in all directions.
- They collide with one other and the container’s sides. (This is how pressure is created inside a can of gas)
Kinetic Particle Theory
This hypothesis, known as the kinetic particle theory, states that all matter is composed of minute particles that move continually and randomly. The term “kinetic particle theory” refers to the kinetic energy of moving particles.
This theory explains how matter changes and describes its current condition. It distinguishes between the characteristics of other states of matter as well. We can better understand how matter behaves by using the particle theory of matter. It also aids in the explanation of why various types of matter have various qualities.
Postulates of Kinetic Particle Theory
- All matter is made of tiny particles. Either single atoms or collections of atoms known as molecules make up these particles.
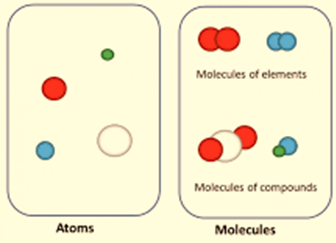
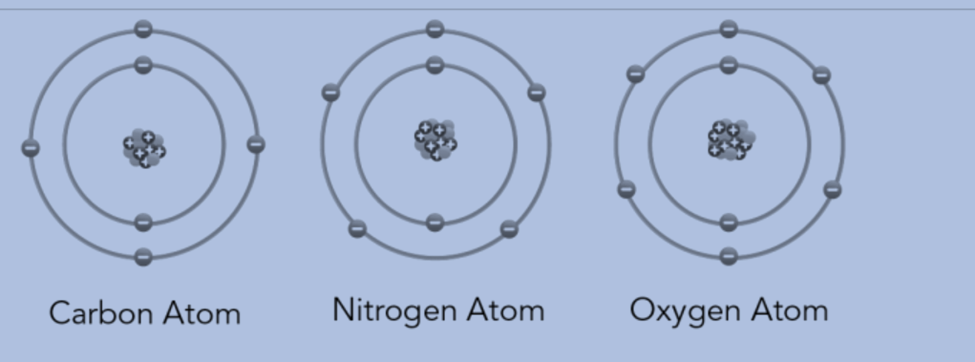
- The atoms of the same element are identical. Different elements’ atoms are distinct. Carbon has the same number of atoms throughout. But unlike the atoms in nitrogen and oxygen, carbon atoms are unique.
- Particles are attracted to one another. The force is especially powerful in certain types of substances, such as diamonds. The force is weaker in other types of materials, such as juice in a glass.
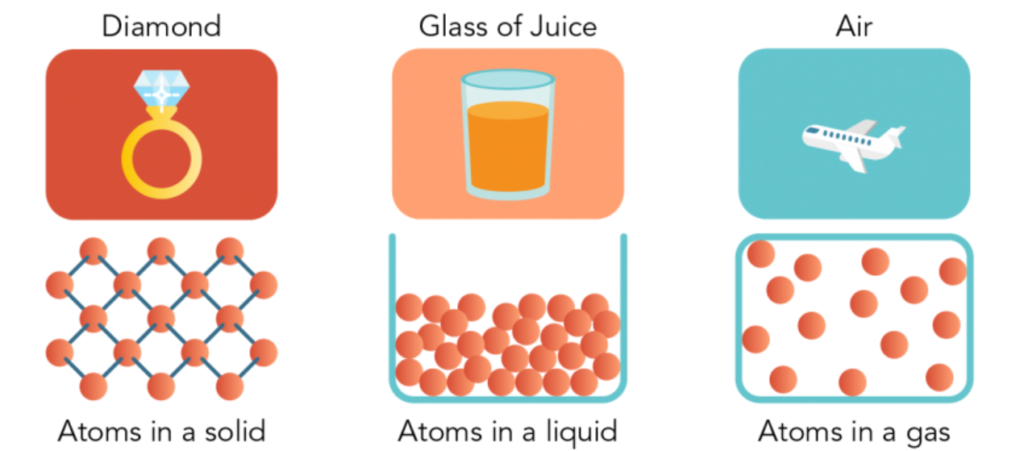
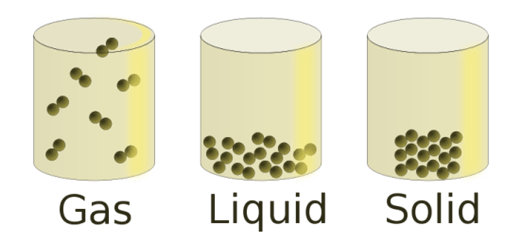
- There are spaces between matter particles. Spaces exist between atoms and molecules. There are wide gaps between them in gas. They are closer together in a liquid. The particles in a solid are so close together that they hardly have room to move.
- Particles move constantly. Any temperature above -273.15 degrees Celsius causes matter particles to travel continuously. The human eye, however, cannot see them move.
Kinetic Molecular Theory of Gases
An assumption that matter is made up of microscopic particles that are always in motion forms the foundation of the kinetic-molecular theory, which explains the states of matter. This theory explains the observable characteristics and behaviors of solids, liquids, and gases are . However, as gases are the subject of kinetic-molecular theory, we will start our in-depth examination with them. The theory specifically pertains to the ideal gas model of a gas. An ideal gas is a hypothetical gas whose behavior exactly matches all of the kinetic-molecular theory’s presumptions. Although they are not perfect in actuality, gases come extremely close to being perfect in most real-world situations.
Assumptions of Kinetic Molecular Theory of Gases
The kinetic-molecular theory makes five fundamental assumptions when it comes to gases:
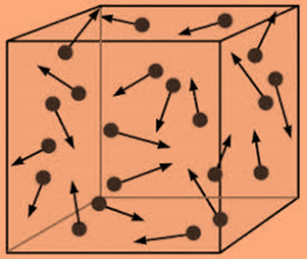
- When compared to their size, the tiny, spherical particles that make up gases are spaced far away from one another. A gas’s constituent particles can be either atoms or molecules. The space between the particles of a liquid or a solid is much, much less than the space between those of a gas. Therefore, the void space between the particles makes up the majority of the volume of a gas. The volume of the particles is actually thought to be little in comparison to the volume of the empty vacuum.
- Gas particles are constantly moving quickly and randomly. Gas particles have a considerable quantity of kinetic energy due to their rapid mobility. Remember that kinetic energy refers to the energy that an object has due to motion. Until they clash with another particle or one of the walls of the gas container, the particles of gas travel in a straight path.
- Elastic collisions occur when gas particles collide with one another and with the container walls. Any collision in which there is no overall kinetic energy loss is an elastic collision. During an elastic collision, kinetic energy may be transmitted from one particle to another, but the combined energy of the colliding particles remains unchanged.
- Between gas particles, there are no forces of attraction or repulsion. Real gas particles that condense to create liquids are due to attractive forces. The particles of a perfect gas lack these attractive forces.
- The temperature of the gas affects the average kinetic energy of gas particles. A speeds of the particle increase as the temperature of a sample of gas rises. Average kinetic energy of a gas’s particles determines its temperature.
Change in State and Kinetic Particle Theory
The kinetic theory of matter explains the interchangeability of solids, liquids, and gases as a result of changes in heat energy.
When an object gets heat, the particles’ velocity increases because they are more energetic. The motion of the particles slows down as they lose energy when it is cools down.
A substance’s physical state can change depending on the surrounding temperature and pressure, for example, ice turns into water and water becomes water vapors. It is possible to reverse state changes. For instance, water can turn into water vapor, which, when cooled, turns back into liquid water. State changes include melting, boiling, freezing, and condensing. All subatomic particles have kinetic energy because they are constantly in motion. Gases have the maximum kinetic energy, while the lowest kinetic energy is in solids.
Heating Curve
Boiling is a physical transition from a liquid to a gas, whereas melting is a physical transition from a solid to a liquid.
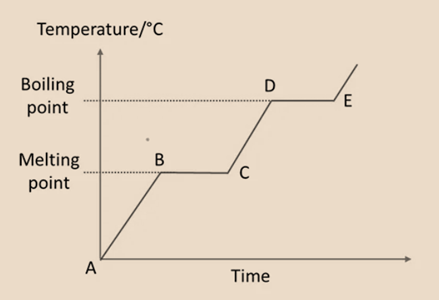
- A – B: The temperature of the solid increases as it gets heat. At this point, the solid’s particles start to gather energy, vibrating more rapidly and dispersing farther apart. The solid begins to dissolve at point B.
- B – C: While the temperature is constant, the substance is now in two states (solid and liquid). To overcome the pulling forces between the particles, heat energy gets absorb.
- C – D: At point C, all of the solid has melted into a liquid. Now that all the particles can slide over one another. As the liquid gets heat continuously, its temperature increases. Liquid begins to boil at point D.
- D – E: The substance exists in both the liquid and gaseous forms at this point, and the temperature is constant. The liquid’s molecule particles absorb heat energy in order to repel each other’s forces.
- All of the liquid has turned into the gas at point E. As the heating progresses past E, the gas’s temperature rises.
Cooling Curve
Condensation is a physical transition from a gas to a liquid, and freezing is a transition from a liquid to a solid.
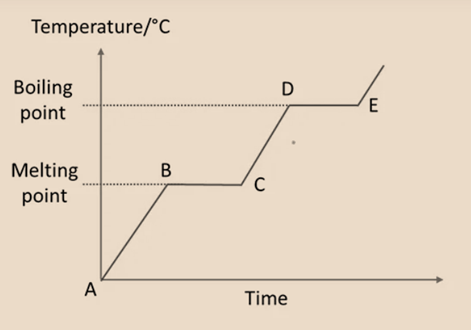
- F – G: The temperature of the gas drops as it cools. The gas’s particles are currently moving quickly and randomly. Gas begins to condense at point G.
- G – H: The material has two states at this point (gas and liquid). At a steady temperature, the particles lose energy and come closer to one another. All gas converts into liquid at point H.
- H – J: The temperature of the liquid is falling as it cools down. Now, the particles overlap one another and liquid begins to freeze at point J.
- J – K: Currently, there are two states of the material (liquid and solid). When a substance transitions from a liquid to a solid state, it loses energy and gets closer together.
- At K, all liquid has solidified into ice. As cooling proceeds, the temperature keeps dropping.
References
- https://chemnotcheem.com/kinetic-particle-theory-notes/
- https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Introductory_Chemistry_(CK-12)/13% 3A_States_of_Matter/13.01%3A_Kinetic_Molecular_Theory
- https://www.achieversdream.com.sg/ s3- kinetic-particle- theory/
- https://sites.google.com/site/olevelexamnotes/chemistry-5072/o-level-chemistry-kinetic-particle-theory
- https://www.savemyexams.co.uk/igcse/chemistry/cie/23/revision-notes/1-states-of-matter/1-1-solids-liquids–gases /1-1-1-kinetic-theory/
- https://letstalkscience.ca/educational-resources/backgrounders/introduction-particle-theory-matter
- https://www.oalevelacademy.com/LessonDetails/olevel/5070/Kinetic-Particle-Theory
