The term “isotope” refers to one of two or more species of atoms of a chemical element that share the same atomic number and position in the periodic table as well as nearly identical chemical behavior, but have different atomic masses and physical characteristics. Every chemical element has one or more isotopes.
The term “isotope” primarily refers to variations in an element’s atomic mass or weight.
It is also defined as variants of a specific element, where these variants have the same number of protons but differ in the number of neutrons in the atom.
Elemental isotopes usually have different masses due to unequal numbers of neutrons. In general, elements with odd atomic numbers have one or two stable isotopes, whereas elements with even atomic numbers have three or more stable isotopes. However, there are some exceptions, such as carbon, helium, and beryllium.
An isotope is typically denoted or identified by the name of the specific element at the beginning, followed by a hyphen and the mass number.
Interesting Science Videos
Isotope Representation
- List an element’s mass number after its name or element symbol. Carbon-12, or C-12, is an isotope with six protons and six neutrons. Carbon-13 or C-16 is an isotope with 6 protons and 7 neutrons.
- It should be noted that the mass number of two isotopes may be the same, despite the fact that they are different elements. You could, for example, have carbon-14 and nitrogen-14.
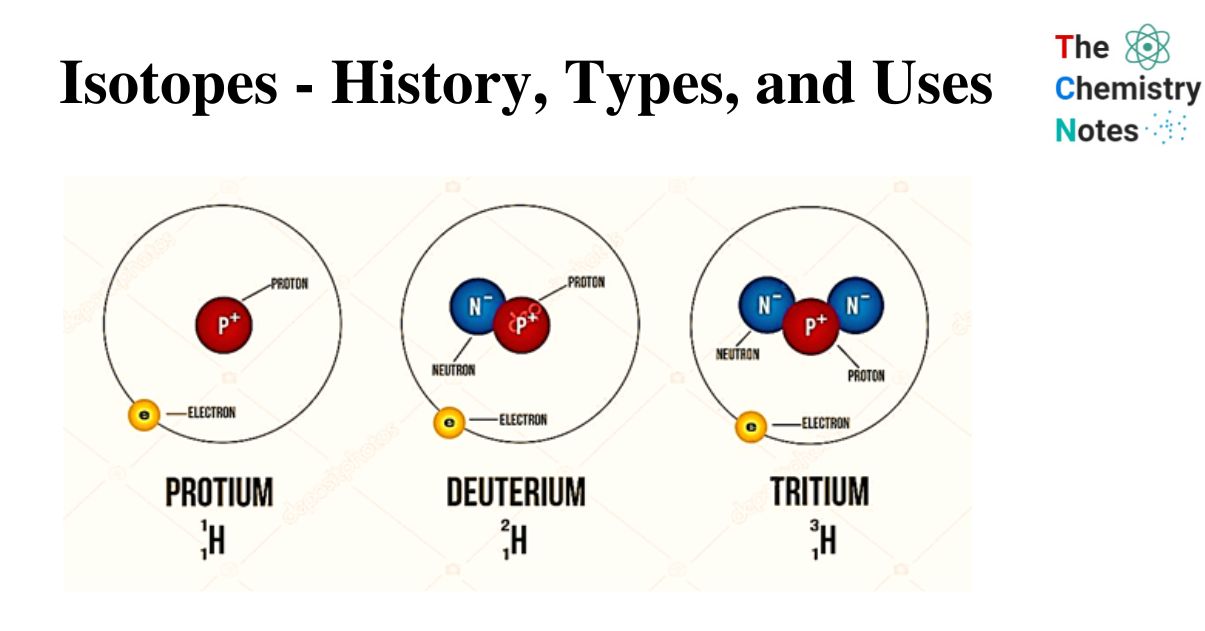
- An element symbol’s mass number can be found in the upper left corner. (Technically, the mass number and atomic number should be stacked in line with each other, but on a computer, they don’t always line up.) For example, the hydrogen isotopes can be written as 11H, 21H, and 31H.
Examples of Isotopes
The chemical symbol (or word) for an isotope is followed by a dash, the mass number, and finally the word.
C-14 (or carbon-14) is a carbon isotope with 6 protons, 6 electrons, and 14 – 6 = 8 neutrons.
It is also possible to write 14C or 146C.
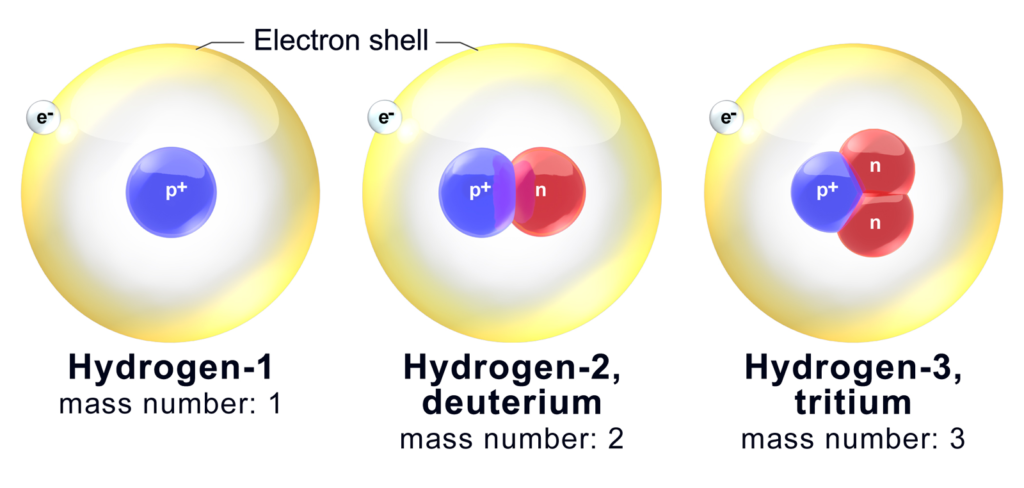
Let us have a look on the isotopes of Hydrogen.
| Isotopes Symbol | Name | |
| Hydrogen-1 | 1H | Protium |
| Hydrogen-2 | 2H | Deuterium |
| Hydrogen-3 | 3H | Tritium |
History of Isotopes
The term “isotope” was coined in 1913 by British chemist Frederick Soddy, who was inspired by Margaret Todd. The word derives its meaning from the Greek words isos (equal) + topos (place). Isotopes of the same element have the same position on the periodic table despite having different atomic weights.
The discovery events of isotopes carries a long history. A few years later, Francis William Aston developed the mass spectrograph (see mass spectrometry), which allowed for the clear confirmation of isotopes in stable elements that were not directly linked to uranium or thorium. His work evolved from the study of positive rays (also known as canal rays), which were discovered by Eugen Goldstein in 1886 and quickly identified as beams of positive ions. Aston discovered that the gaseous element neon produced two positive rays as a student in J.J. Thomson’s laboratory. The masses of ions in the heavier ray were about two units, or 10%, greater than those in the lighter ray.
Aston had to build a much more precise instrument than anyone else at the time in order to prove that the lighter neon had a mass very close to 20 and that the heavier ray was indeed neon and not some kind of spurious signal. By 1919, he had done so and had convincingly argued for the existence of neon-20 and neon-22. Information from his and other laboratories accumulated quickly in the years that followed, and by 1935, all but a few elements’ main isotopes and relative proportions were known.
Frederick Soddy
Kasimir Fajans (1887-1975) and Soddy reached the same conclusion in 1913. (1877-1956). The chemically identical atoms with different isotopic masses were referred to as pleiads by the Fajans, but Soddy referred to them as isotopes, which is the name we now use. Soddy argues against Fayan’s idea that isotopes are distinguished by electronic changes (for example, 90U4+ would be identical to 90Th2+). Neutrons were unknown at the time, and atoms were thought to be pairs of positively and negatively charged particles.
Frederick Soddy received the Nobel Prize in Chemistry in 1921 “for his contributions to our understanding of the chemistry of radioactive substances, as well as his investigations into the origin and nature of isotopes.”
Same Chemical Characteristics of Isotopes
- Isotopes of the same element have the same chemical properties. This is due to the fact that they have the same number of electrons in their outer shells and, as a result, the same electronic configuration, which determines an atom’s chemistry.
- The number of neutrons, which are neutral particles within the nucleus that only add mass, differs between isotopes.
- The mass difference influences physical properties such as density, boiling point, and melting point.
- Because isotopes have the same appearance, a sample of C-14 would look the same as a sample of C-12.
Water made from deuterium oxide is known as ‘heavy’ water because it has a relative formula of mass 20 as opposed to 18 for water, making it 20% heavier, but it would look, taste, and feel the same. However, drinking it is not a good idea because it is toxic and interferes with biochemical reactions in your cells!
Types of Isotope
Isotopes can be classified as either stable or radioactive. As a result, radioactive isotopes are frequently referred to as radioisotopes or radionuclides.
Stable isotopes or stable nuclides are isotopes that do not decay radioactively.
According to the findings, there are approximately 339 naturally occurring nuclides or isotopes on the planet. There are 286 primordial nuclides in this, which are thought to have existed since the formation of the Solar System.
Radioactive Isotope
The term radioactive isotope refers to any of several species of the same chemical element with different masses whose nuclei are unstable and dissipate excess energy by spontaneously emitting radiation in the form of alpha, beta, and gamma rays.
Only a small percentage of isotopes are known to be indefinitely stable. All of the others disintegrate spontaneously, releasing energy through processes known as radioactive decay. Each radioactive isotope “parent” eventually decays into one or a few stable isotope “daughters” specific to that parent. The radioactive parent tritium (3H, or hydrogen-3), for example, always emits an electron and transforms into the daughter helium-3 (3He).
Under normal conditions, each radioactive isotope disintegrates at a predictable and predictable rate. As a result, without replenishment, any radioactive isotope will eventually vanish. Some isotopes, on the other hand, decay so slowly that they remain on Earth even after more than 4.5 billion years since the last significant injection of freshly synthesized atoms from a nearby star. Every chemical element contains at least one radioactive isotope. For example, the lightest element, hydrogen, has three isotopes with mass numbers 1, 2, and 3. Only hydrogen-3 (tritium) is radioactive, while the other two are stable. There are over 1,000 radioactive isotopes of each element known. Around 50 of these are found in nature; the rest are created artificially as direct products of nuclear reactions or as radioactive descendants of these products.
Parent and Daughter Isotope
When radioisotopes undergo radioactive decay, the initial isotope and the resulting isotope may differ. The initial isotope is referred to as the parent isotope, and the atoms produced by the reaction are referred to as daughter isotopes. There may be more than one type of daughter isotope produced.
Parent isotopes are isotopes of one chemical element that can undergo radioactive decay to form another chemical element’s isotope. Daughter isotopes, on the other hand, are the result of parent isotope radioactive decay. That is the primary distinction between parent and daughter isotopes.
When U-238 decays into Th-234, for example, the uranium atom is the parent isotope, while the thorium atom is the daughter isotope.
Uses of Isotopes
- Carbon-14, a carbon isotope, is used in carbon dating. This carbon isotope exists in the atmosphere as radioactive carbon. The amount of carbon-14 found in fossils aids palaeontologists in determining the age of the fossils.
- Krypton-85 indicator lights are used to determine the thickness of thin plastics and sheet metal, rubber, textiles, and paper, as well as to measure dust and pollutant levels in appliances such as clothes washers and dryers, stereos, and coffeemakers.
- Used in explosive detection, electronic device voltage regulators and current surge protectors, and electron capture detectors in gas chromatographs.
- Uranium isotopes are widely used in nuclear reactors. Nuclear reactors use U-235 as a fuel.
- Radioactive isotopes are employed in medicine. They are used to detect tumors, blood clots, and other abnormalities. Arsenic-74, an arsenic isotope, is used to detect the presence of a tumor. Sodium-24, on the other hand, is used to detect blood clots.
- Thallium-204 The levels of dust and pollutant on filter paper are measured, as well as the thickness of plastics, sheet metal, rubber, textiles, and paper.
- Cadmium-109 Metal alloys are analyzed for stock control and scrap sorting.
- Carbon-14 is a valuable research tool. Contributes to research by ensuring that potential new drugs are metabolized without the formation of harmful by-products. It is used in biological research, agriculture, pollution control, and archaeology.
- The carbon isotope cobalt (cobalt-60) is used in cancer treatments.
- The carbon isotope iodine (Iodine-131) aids in the treatment of goitre.
- Nickel-63 Used in explosive detection, electronic device voltage regulators and current surge protectors, and electron capture detectors in gas chromatographs.
For pictorial understanding have a look in the video.
References
- https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Atomic_Theory/Isotopes/Isotopes_II
- https://www.savemyexams.co.uk/igcse/chemistry/cie/23/revision-notes/2-atoms-elements–compounds/2-1-atomic-structure–the-periodic-table/2-1-4-isotopes/
- https://www.thoughtco.com/definition-of-isotopes-and-examples-604541
- https://byjus.com/chemistry/periodic-table-isotopes-element/
- https://byjus.com/chemistry/practical-uses-of-isotopes/
- https://www.toppr.com/guides/chemistry/structure-of-atom/isotopes/
- https://www.sciencedirect.com/topics/earth-and-planetary-sciences/isotope
- https://link.springer.com/book/10.1007/978-1-4612-3498-2
- https://www.sciencedirect.com/topics/engineering/parent-isotope

