Positive and negative ions are arranged alternately in three dimensions in ionic lattices. The term “giant ionic structures” is occasionally used to describe compounds with ionic lattices. Ionic bonds maintain the integrity of the ionic lattice. Ionic bonds between ions are depicted as straight lines in three-dimensional models. In most ionic compounds, the anions are much larger than the cations, and the anions form the crystal array. In the spaces between the anions, the smaller cations are found.
Theoretically, ions are charged, incompressible, nonpolarizable spheres. Ions attempt to closely encircle themselves with as many ions with the opposite charge as possible. The cation is typically sized in the packing arrangement so that the anions can surround it without touching it.
The ratio of cations to anion must correspond to the compound’s stoichiometry. For instance, the array of chloride anions in the lattice for MgCl2 must only contain half as many magnesium ions.
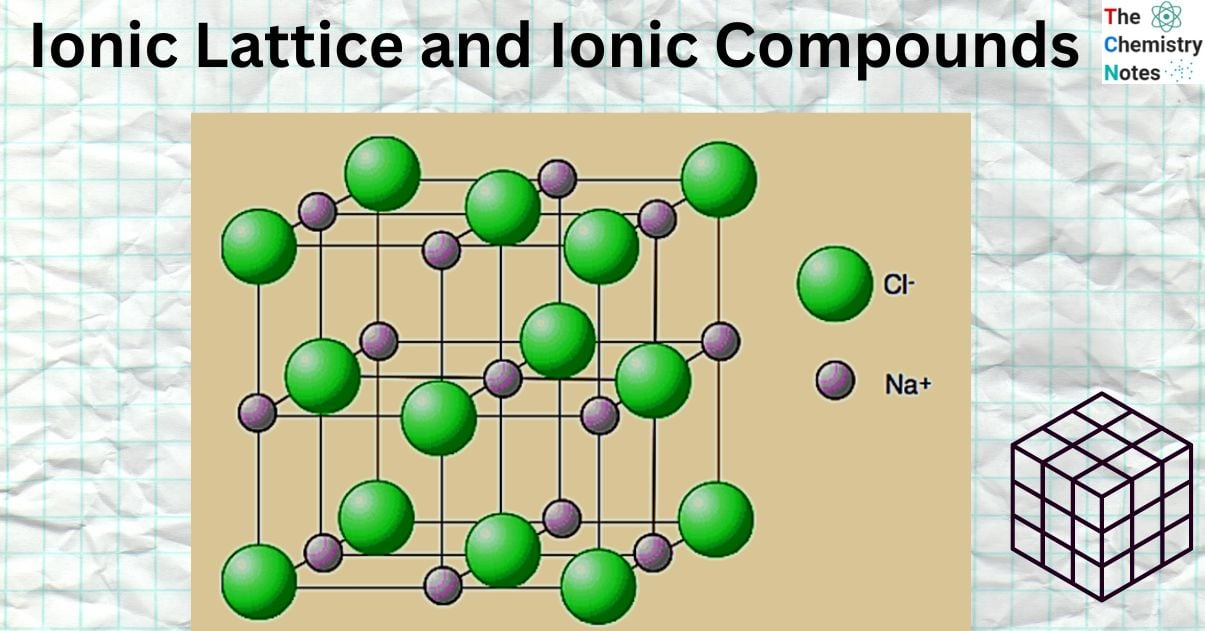
Ionic Lattice
An ionic lattice is a three-dimensional regular repeating arrangement of ions. Positive ions surround every negative ion, and negative ions surround every positive ion. The actual ion packing structure is determined by the relative sizes and charges of the ions.
The relative sizes of the ions present determine the type of lattice that forms. Magnesium oxide and sodium chloride both have cubic ionic lattices. Six chloride ions with opposing charges surround each sodium ion in sodium chloride. Compared to sodium ions, chloride ions are significantly larger. The sodium ions are as close to the chloride ions as possible by fitting into the spaces between them.
Similarly, the lattice structure of magnesium oxide is identical to that of sodium chloride. Oxide ions replace the chloride ions, while magnesium ions replace the sodium ions.
Lattice Energy
The forces of attraction between the positive and negative ions determine the lattice energy. This electrostatic force is influenced by two factors:
- The magnitude of the charge on the ions
- The distance between the ions (the sum of the ionic radii)
The equation for determining the electrostatic force of attraction is:


In summary, the force of attraction between two ions is proportional to the product of their magnitudes and inversely proportional to the square of the distance between them.
The energy needed to overcome the forces of attraction holding the ions together in the lattice, or the lattice enthalpy, determines how strong a lattice is. A mole of a crystal lattice must be split into a finite number of gaseous ions, which requires a certain amount of energy. For instance, the formula for sodium chloride lattice enthalpy is
NaCl(s) ⟶ Na+(g) + Cl–(g) ΔH = +790 kJ mol-1
Ions with a double charge produce lattices with a much higher lattice enthalpy.
Ionic Compounds
When an element made up of atoms that readily lose electrons, such as a metal, reacts with an element made up of atoms that readily gain electrons, such as a nonmetal, an electron transfer typically takes place, leading to the formation of ions. The electrostatic attraction (ionic bonds) between the ions of opposite charge present in the compound stabilizes the compound created by this transfer.
For instance, the compound NaCl is made up of sodium ions and chloride ions in the ratio of one Na+ ion for every Cl– ion when each sodium atom in a sample of sodium metal gives up one electron to form a sodium cation, Na+, and each chlorine atom in a sample of chlorine gas accepts one electron to form a chloride anion, Cl–. Similar to this, each calcium atom can transfer one electron to each of the two chlorine atoms to form CaCl2, which is made up of one Ca2+ ion and two Cl– ions.
Ionic compounds are those that contain ions and are held together by ionic bonds. The compound is frequently ionic when a metal is combined with one or more nonmetals. For the majority of the compounds, this rule is effective at predicting the formation of ionic compounds. But it’s not always the case (for example, aluminum chloride, AlCl3, is not ionic). Ionic compounds are easily identified due to their properties.
Properties of Ionic Compounds
- They are hard. The ions are held together by powerful attractive forces.
- They are brittle. When impacted in the same direction as the layers of ions, ionic crystals may break apart. The force of the blow may displace the layers of ions, causing ions with the same charge to unite. The crystal splits along these cleavage planes as a result of the attraction between thousands of ions in the layers that have the same charge.
- Due to the attraction between the numerous oppositely charged ions present in the lattice, which acts in all directions and strongly bonds them together, they have high melting points and boiling points. The charge density of the ions raises the melting and boiling points. As a result, sodium chloride, Na+Cl–, has a lower melting point (801 °C) than magnesium oxide, Mg2+O2- (2852 °C). This is due to the fact that doubly charged ions exhibit a stronger electrostatic attraction than singly charged ions of comparable size.
- Ionic compounds, rather than amorphous solids, form crystal lattices. Although molecular compounds can form crystals, these crystals are typically softer than ionic crystals and they can also take on other shapes.
- Most of them are water-soluble, and they only conduct electricity when molten or in solution.
- Ionic compounds typically have enthalpies of fusion and vaporization that can be 10 to 100 times higher than those of the majority of molecular compounds.
- Although they conduct electricity when molten or in an aqueous solution, ionic solids do not conduct electricity very well because the ions are so tightly bound to each other.
Uses of Ionic Compounds
- Ionic compounds have a long history of being used in numerous different ways.
- Many minerals are ionic in nature.
- Over 8000 years ago, humans first processed common salt (sodium chloride), using it as a food seasoning and preservative. Today, it is also used in manufacturing, agriculture, water treatment, de-icing roads, and many other applications.
- Many ionic compounds are so commonly used in daily life that their chemical identities are obscured by their common names. Borax, calomel, milk of magnesia, muriatic acid, oil of vitriol, saltpeter, and slaked lime are a few examples of this.
- Numerous collinear properties, such as raising the osmotic pressure, lowering the freezing point, and elevating the boiling point are all impacted by the solute concentration.
- Solid ionic compounds have been used for a very long time as paint pigments because they are resistant to organic solvents but sensitive to acidity or basicity.
- Ionic compounds are frequently employed in chemistry as precursors for high-temperature solid-state synthesis.
References
- https://www.bbc.co.uk/bitesize/guides/zy98msg/revision/3
- https://www.savemyexams.co.uk/igcse/chemistry/cie/23/revision-notes/2-atoms-elements–compounds/2-2-ions–ionic-bonds/2-2-2-ionic-bonds–lattice-structure/
- https://courses.lumenlearning.com/chemistryformajors/chapter/molecular-and-ionic-compounds-2/
- https://en.wikipedia.org/wiki/Ionic_compound
- Atkins, Peter; de Paula, Julio (2006). Atkins’ physical chemistry (8th ed.). Oxford: Oxford University Press. ISBN 978-0-19-870072-2.
- Barrow, Gordon M. (1988). Physical chemistry (5th ed.). New York: McGraw-Hill. ISBN 978-0-07-003905-6.
- Ashcroft, Neil W.; Mermin, N. David (1977). Solid state physics (27th repr. ed.). New York: Holt, Rinehart and Winston. ISBN 978-0-03-083993-1.
