An infrared Spectrophotometer (or infrared spectrometer) determines the chemical composition of liquid or solid polymer sample. The infrared spectrum of different chemical composition will differ according to their structure and hence, the reading of infrared spectrum absorptions allows us to identify the unknown composition of the samples.
A spectrophotometer consists of two components: a spectrometer and a photometer. The spectrometer generates light of any wavelength, whereas the photometer measures light intensity. The liquid or sample is put between the spectrometer and the photometer in the spectrophotometer. The photometer detects how much light travels through the sample and sends a voltage signal to the display.
What is Infrared Spectrophotometer?
An infrared spectrophotometer is an analytical tool for identifying compounds such as organic polymers. It is also known as an infrared spectrometer, which generates an infrared spectrum, is used to carry out the process or technique of infrared spectroscopy.
As infrared light travels through a sample, infrared spectrophotometers measure the relative quantity of energy as a function of wavelength/frequency. As a result, the chemical structures of various samples will exhibit changes in the IR absorption spectra, allowing for sample identification.
Instrumentation of Infrared Spectrophotometry
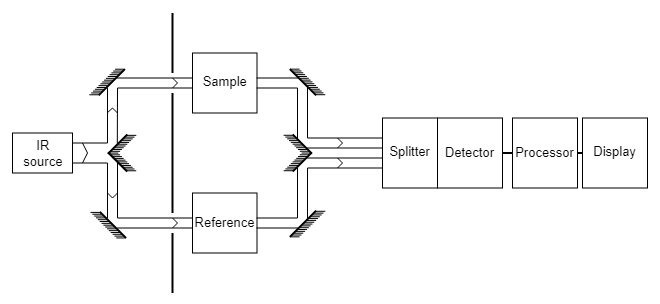
Infrared spectrometers are classified into several varieties, however they share similar characteristics. All of them have a source that emits all of the relevant infrared radiation.
A detector is required for all spectrometers. Detectors convert radiation energy into an electrical signal, which may then be amplified and processed to produce a spectrum.
Thermal detectors detect the heating impact of radiation and respond to all wavelengths equally. Thermocouples, bolometers, and pyroelectric detectors are among the examples.
Photodetectors are detectors that use photon energy to release bound electrons in the detector material. Unlike thermal detectors, photodetectors do not respond to all wavelengths but have a long-wavelength limit where the photon does not have enough energy to excite the electrons. One example is the photoconductive detector, in which photon energy absorption drives bound electrons to free states.
A graph showing infrared light absorbance (or transmittance) on the vertical axis vs. frequency, wavenumber, or wavelength on the horizontal axis can be used to depict an IR spectrum. Infrared spectra often employ reciprocal centimeters, denoted by the sign cm-1. Units of IR wavelength are generally provided in micrometers (previously termed “microns”), symbol m, which are reciprocally linked to the wavenumber.
The spectrometer must have some way of measuring the radiation between the source and the detector so that intensity may be derived for each wavelength resolution element. Monochromators and interferometers, two very distinct types of devices, are employed. Dispersive instruments employ monochromators with gratings or prisms, whereas Fourier transform instruments use interferometers.
Advantages of Infrared Spectrophotometer
- The general infrared spectrum refers to the infrared spectrum greater than 760nm, which is the most often utilized spectral area of organic substances and can assess a wide range of sample circumstances (gas, liquid, solid).
- Fast, low sample volume (a few micrograms to a few milligrams), robust characterisation (different compounds have their own distinctive infrared spectrum).
- This is capable of assessing multiple states (gas, liquid, solid) without destroying the sample define infrared spectroscopy.
Uses of Infrared Spectrophotometer
- Infrared (IR) spectrometers are used by chemists to test a sample’s reaction to infrared light.
- To measure absorbance, the instrument delivers a series of IR wavelengths through the sample. Because the vibrational and rotational frequencies of atoms linked to each other are the same as the frequencies of IR radiation, IR spectroscopy is also known as vibrational or rotational spectroscopy.
- Since a substance’s IR spectrum acts as a unique “fingerprint,” IR spectrometers are used to identify unknown chemicals or to validate their identity.
- As a result, there are several benefits, including a better signal-to-noise ratio, improved resolution, higher throughput, and a shorter wavelength limit.
- Infrared spectrophotometers have a wide range of applications, including environmental, medicinal, and petrochemical.
Watch the video of Infrared Spectrophotometer in Lab
References
- Vogel’s Textbook of Quantitative Chemical Analysis sixth edition by J Mendham, RC Denney, JD Barnes, M Thomas
- https://www.labcompare.com/Spectroscopy/116-Infrared-Spectrophotometer-IR-FTIR-Spectrometer/
- https://sciencing.com/spectrometer-5372347.html
- H M Pollock and S G Kazarian, Microspectroscopy in the Mid-Infrared, in Encyclopedia of Analytical Chemistry (Robert A. Meyers, Ed, 1-26 (2014), John Wiley & Sons Ltd,

