The p-block elements with higher atomic numbers [e.g., Ga, In, Tl (Gr IIIA), Ge, Sn, Pb (Gr IVA), and As, Sb, Bi (Gr VA)], display two oxidation states they are:
Interesting Science Videos
Higher oxidation states (Group oxidation states)
These are equal to the number of valence electrons present in ns and np orbitals. These oxidation states are obtained when all the electrons in ns and np orbitals are involved in bonding.
Lower oxidation states
These are equal to the group oxidation state minus two (i.e., G-2). These results when only the electrons in np orbitals are participating in bonding and ns2 remains paired.
As one goes down to a group in the periodic table the lower oxidation state tends to be more stable. This is referred to as the inert pair effect. For example, in group IIA, Thallium the heaviest member in the group is quite unstable in the +3 state and is readily reduced to give a more stable +1 state. Similarly, Pb+2 is more stable than Pb+4 in group IVA.
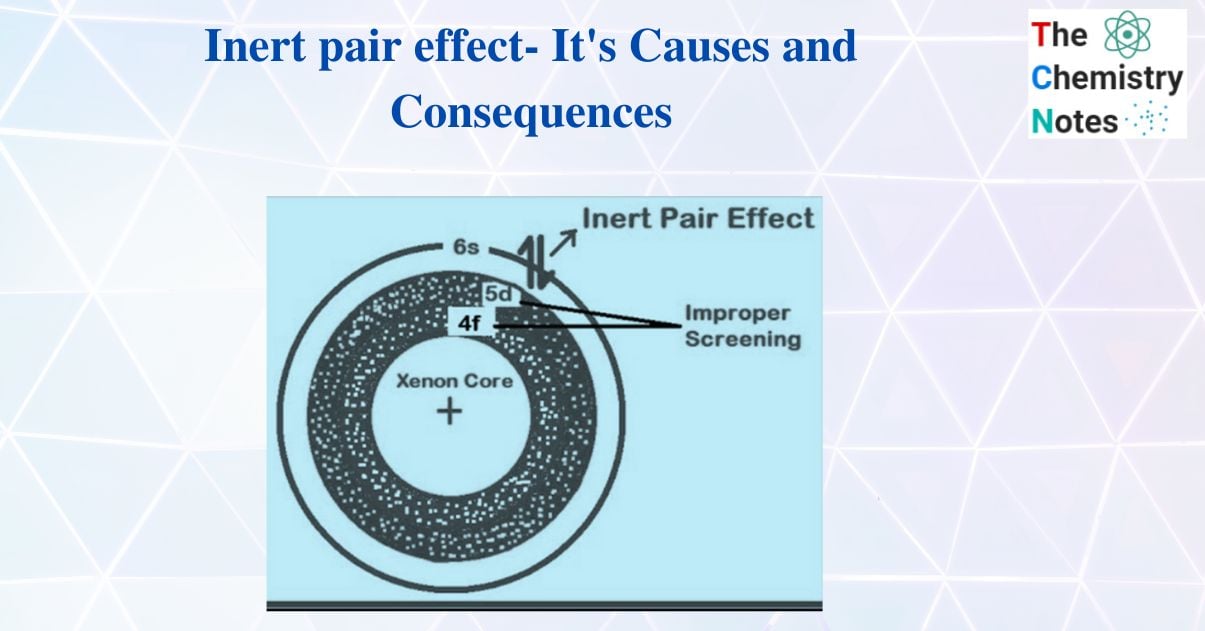
The term inert pair effect was first proposed by Nevil Sidgwick in 1927. It is the reluctance of s-electrons to become unpaired or participate in covalent bonding. Only p orbitals electrons are involved in bond formation in this effect.
Cause of inert pair effect
The explanation for this effect is described in terms of bond energy. On the one hand, energy is required to uncouple the s-electrons, but energy is also released during bond formation. If the energy released is enough to unpair the s- electrons, they will participate in bond formation; otherwise, they will not.
For example, the valence shell configuration of the atoms of group III A is ns2, np1. Since the np electrons are farther away from the nucleus than the ns electrons, they can easily be removed or excited to a higher oxidation state than the ns2 electrons. As a result, the ns electrons are thought to be inert, i.e., the ns2 electrons remain paired and do not participate in bonding, particularly in the heaviest element. So, if the energy required to unpaired ns-electrons exceeds the energy that evolves when they form bonds, then the s-electron will remain paired.
Inert pair effect regarding the group IIIA (13) elements
Group III A (13) elements have outer electronic configuration ns2 np1 and so they should have trivalent. But in some cases, the element also exhibits a monovalency. The monovalency is explained by the tendency of the s-electron in the outer shell to remain paired or not participate in bonding, this is called the inert pair effect.
There is an increasing tendency to form univalent compounds in the descending group. For example, the compounds with Ga (I), In, and Tl(I), are known. Compounds with Ga and In, +1 oxidation states are less stable than compounds with +3 oxidation states. However, compounds with Tl +1 oxidation state, are more stable than compounds with Tl +3 oxidation state.
Inert pair effect based on group IV A (14) elements
Group IV A (14) elements have their outer electronic configuration ns2 np2. When all four valence electrons are lost, we get the element in a +4 oxidation state i.e., M+4 cations are formed. The stability of the M+4 cation decreases from Ge4+ to Pb 4+. When only two np electrons from ns2 and np2 are lost we get the elements in a +2 oxidation state. I.e., M+2 cations are formed. In this case, the S-electrons in the outermost shell remain paired and do not participate in bonding. The stability of the +2 oxidation state increases on descending the group. So, the Pb+2 compounds are more stable than the Pb4+ compounds. However, compounds of Ge2+ and Sn 2+ are less stable than those of Ge4+ and Sn4+.
Inert pair effect based on group V A (15) elements
The valence shell configuration of group VA (15) elements is ns2 np3 which suggests that these elements show +3 and +5 oxidation states. When only three np electrons are involved in the bonding, a +3 oxidation state is obtained whereas when all three np and two ns electrons are involved in bonding +5 oxidation state is obtained.
Based on the inert pair effect it can be explained that the stability of the +3 oxidation state increases while that of the +5 state decreases as we move down to the group from N to Bi. The lowermost element Bi prefers to show a +3-oxidation state. Thus, Bi in a +3-oxidation state is more stable than when it is in a +5 oxidation state. Consequently, Bi forms only trihalides (BiCl3) and not pentahalides.
Consequences of inert pair effect
Many physical and chemical properties of the corresponding elements change as a result of the inert pair effect. The change in properties of elements and compounds caused by the inert pair effect is further discussed below.
1. Stability of compounds and elements
The inert pair effect alters the stability of elements and compounds. For example, PbI4 is unstable while SnI4 is stable. In Sn, the +4-oxidation state is more stable. So SnI4 is stable. Whereas in Pb, the +2-oxidation state is more stable. So PbI4 is unstable and it decomposes into more stable compounds PbI2 as follows.
PbI4 → PbI2 + I2
2. Variable valency of elements
The inert pair effect causes the variable valency of elements. For example both In and Tl show +3 and +1 oxidation states.
3. Effect on oxidizing and reducing properties of compounds
Compounds’ oxidizing and reducing properties are influenced by the inert pair effect.
For example, Compounds of Pb4+ act as strong oxidizing agents. As lead is a group IV A element in the periodic table and has outer electronic configuration ns2 np2. When all four valence electrons are lost Pb4+ ion is formed. When only two np electrons are lost, we get a Pb2+ ion. The stability of the +2 oxidation state increases descending the group. Lead is a lowermost element of group IV A. Inert effect is more pronounced in Pb and therefore Pb2+ compounds are more stable than Pb4+ compounds. Hence the compounds of Pb4+ readily reduced into those of Pb2+. Therefore, the compounds of Pb4+ act as strong oxidizing agents.
Pb 4+ compounds (less stable) → Pb 2+ (More stable) + 2 e-
4. Effect on melting point and boiling point of compounds
In general, the melting and boiling points of group 16th elements increase as their atomic number. The ‘Po’-element now has the greatest number of ‘d’ and ‘f’ electrons. As a result, the inert pair effect becomes more effective for ‘Po’ than ‘Te’. As a result, Po has a lower interatomic vander Waals force of attraction than Te. Consequently, polonium has a lower melting and boiling point than tellurium.
Limitation of inert pair effect
I has some limitations. For example, the ionization enthalpy value of group 13 elements
| Ionization enthalpy (KJ/mol⁻¹) | Boron (B) | Aluminum (Al) | Gallium (Ga) | Indium (In) | Thallium (Tl) |
| ∆H₂+∆H₃ | 6086 | 4560 | 4941 | 4524 | 4848 |
Moving from up to down, the ionization enthalpy decreases. However, some anomalies have been observed, such as an increase in ionization enthalpy from Al to Ga and In to Tl. Here, the d-block contraction influences Ga’s ionization energy, whereas the relativistic effect caused by poor d and f orbital shielding is responsible for Tl’s high ionization enthalpy. These anomalies cannot be explained by the inert pair effect.
References
- Lee J. D. (1977). A new concise inorganic chemistry (3d ed.). Van Nostrand Reinhold. Retrieved October 20 2022 from https://archive.org/details/newconciseinorga00leej.
- https://byjus.com/question-answer/what-is-inert-pair-effect-explain-with-an-example/
- http://www.lscollege.ac.in/sites/default/files/e-content/TDC%20PART%20I%20%2CIntoduction%20of%20p-%20block%20and%20inert%20pair%20effect.pdf
- https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Map%3A_Inorganic_Chemistry_(Miessler_Fischer_Tarr)/08%3A_Chemistry_of_the_Main_Group_Elements/8.06%3A_Group_13_(and_a_note_on_the_post-transition_metals)/8.6.02%3A_Heavier_Elements_of_Group_13_and_the_Inert_Pair_Effect
- https://unacademy.com/content/jee/study-material/chemistry/oxidation-states-and-inert-pair-effect/
- https://kgghosh1990.medium.com/inert-pair-effect-definition-examples-cause-and-consequences-ca8ad5024c3e
- https://protonstalk.com/p-block-elements/inert-pair-effect/
