High performance liquid chromatography (HPLC) technique involves the separation of analytes dissolved in the mobile phase using a particular interaction with a stationary phase.
In general, it is a greatly enhanced form of column chromatography. A solvent is forced through a column at high pressures rather than being permitted to flow through it under gravity. As a result, it is considerably quicker.
It employs a smaller particle size for the column packing material, resulting in a substantially larger surface area for interactions between the stationary phase and the molecules moving through it. This also makes it possible to separate the mixture’s components considerably more effectively. The employment of different detection techniques is the other significant advancement over column chromatography. These processes are also exceedingly sensitive and highly automated.
Interesting Science Videos
History of High Performance Liquid Chromatography (HPLC)
Martin and Synge described the discovery of liquid-liquid partition chromatography in 1941,
and also provide the foundation for liquid-liquid chromatography and high-performance performance liquid chromatography. They also created the idea of the Height Equivalent to the Theoretical Plate, which is now used as a standard for determining the effectiveness of chromatography.The mobile liquid phase in classical column liquid chromatography moves slowly through the column due to gravity. Low column efficiency and lengthy separation durations are two general characteristics of this approach. Due to Kirkland and Huber’s discovery of HPLC, there has been a very noticeable revival of interest in the technology of liquid column chromatography since about 1969.
In this technique, The eluent is injected through the column at a high flow rate in HPLC, which uses tiny diameter columns (1-3mm) with support particle sizes around 30m. According to research, HPLC separation can be accomplished approximately 100 times faster than with traditional liquid chromatography. These liquid chromatography devices also overcome the effect of increased liquid viscosities relative to gas viscosities by working at high pressures and providing analysis times similar to GLC.
Types of High Performance Liquid Chromatography (HPLC)
The different types of HPLC methods are as follows:
I. Based on modes of chromatography
1. Normal phase mode:
In normal phase mode, a non-polar solvent, such as hexane, the column filled with silica particlesis used. As the polar chemicals adhere to the polar silica for a longer period of time than non-polar compounds do, non-polar compounds in the mixture will move through the column more quickly.
2. Reverse phase mode:
Reverse phase mode utilizes the column packed with silica particles that have been modified to become non-polar by adding long hydrocarbon chains ( 8-18 C atoms) to their surface. Then a mixture of polar solvents like a water-alcohol solution is used. Since the polar solvent and the mixture’s polar molecules are strongly attracted to one another, polar compounds will move through the column more quickly. The non-polar molecule interacts more easily with the hydrocarbon groups because they are less soluble in the aqueous mobile phase components. As a result, The movement of non-polar molecules through the column is slow.
II. Based on principle of separation
1. Adsorption chromatography: This involves the retention of analytes through reversible binding to a stationary phase.
2. Ion exchange chromatography: Ion chromatography, is a method that separates ions and polar compounds according to their affinity for ion exchangers.
3. Ion pair chromatography: It is a successful reversed-phase liquid chromatography technique that allows ions in solution to be “paired” or neutralized and separated as ion pairs.
4. Size exclusion(or)Gel permeation chromatography: It is a method for separating molecules in solution based on their size and, in some situations, molecular weight.
5. Affinity chromatography: The process of separation that involves the biospecific ligand interaction to separate the analyte.
6. Chiral phase chromatography: It is chromatographic techniques for separating chiral chemical enantiomers
III. Based on the elution technique
1. Isocratic separation:
It maintains the mobile phase’s mobile composition throughout the elution process.
2. Gradient separation:
In this process, the mobile phase’s composition regularly changes in each step of the elution,.
D. Based on the scale of operation
1. Analytical HPLC:
Systems utilized in an analytical HPLC run are used to determine a compound’s quality and quantity. In addition to the manufacturing, healthcare, and research fields, analytical HPLC systems are employed in the food safety and pharmaceutical industries.
2. Preparative HPLC
Preparative HPLC is used in the chemical and pharmaceutical industries, as well as in biotechnology and biochemistry, for the separation and purification of valuable compounds.
IV. Based on the type of analysis
1. Qualitative analysis
Qualitative analysis compares a sample component’s retention duration to that of a standard sample to identify it.
2. Quantitative analysis
Quantitative analysis is carried out utilizing a calibration curve. For quantification, a calibration curve is made using the concentration ratio and peak area ratio.
Working principle of HPLC
Adsorption is the primary separating principle of HPLC. The sample molecule and the column packing particles interact in numerous ways, whether chemically or physically when a mixture of components is added to the column. Their respective affinities to the stationary phase determine how they move. The component that is more attracted to the stationary phase moves more slowly. The component that has a lower affinity for the adsorbent moves more slowly.
In the HPLC method, a small amount of sample is introduced into a tube that is filled with tiny particles. When liquid is driven through the column by a high pressure supplied pump, individual sample components are moved along the packed tube.
The column packing separates these components by involving numerous chemical and physical interactions between their molecules and the packing particles.
Instrumentation of High Performance Liquid Chromatography (HPLC)
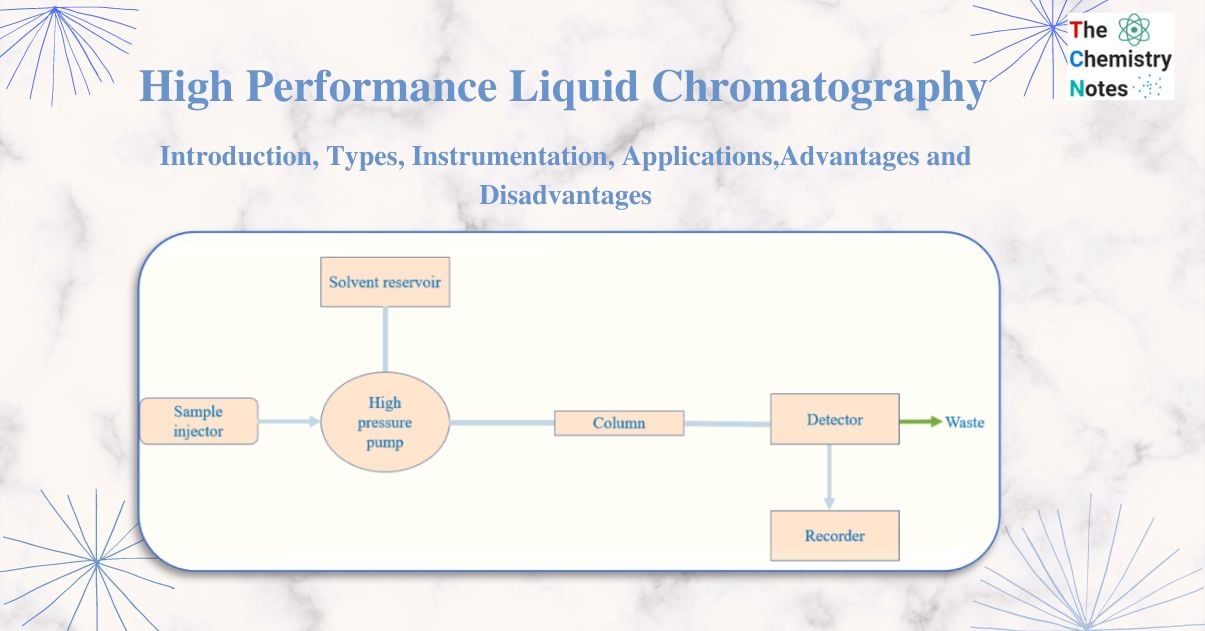
The major components of an HPLC are
1. Solvent delivery system
2. Pumps
3. Sample injection system
4. Column
5. Detectors
6. Recorders
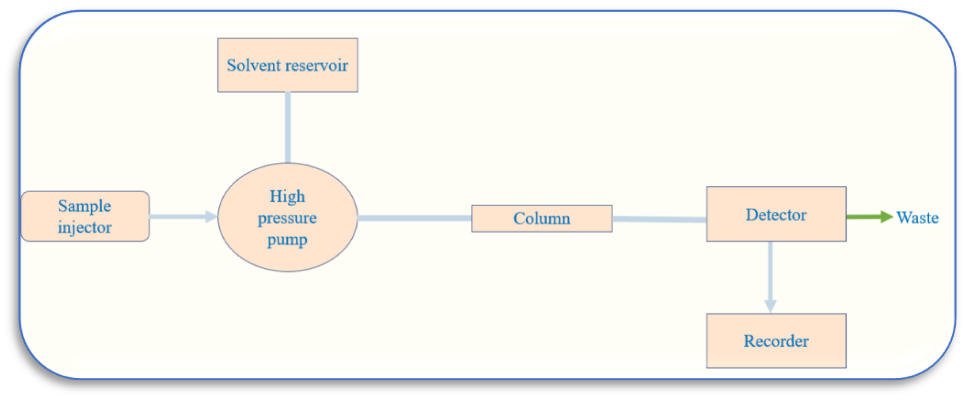
Solvent delivery system (Solvent resorvoir)
It is necessary to move the solvents or mobile phases through the column under high pressure, between 1000 and 3000 psi. In HPLC, the mobile phase selection is crucial, because the mobile phase’s eluting power is influenced by its overall polarity, the stationary phase’s polarity, and the nature of the sample. Highly viscous solvents are typically avoided because they take longer to move through the column, which causes peak widening, poor resolution, and high pressure to push the solvent through the column.
The pump system (High pressure pump)
A mobile phase is pushed through the column by the pump at a specific flow rate. Pumps also supply the column with a steady flow of the mobile phase at a constant pressure.
Sample injection system
The liquid sample is injected into the mobile phase’s flow stream using an injector. The sample is injected continuously into the mobile phase stream using injectors. Additionally, the injector needs to be able to withstand the high liquid system pressure. Similarly,to maintain a high level of accuracy, injection must be inert and repeatable.
Column
The column is the heart of the chromatograph and is usually made of stainless steel to withstand high pressure caused by the pump to move the mobile phase through column packing. Due to its particle form, surface characteristics, and weak structure, silica gel is typically used as the column packing since it provides effective separation. The sample component of interest is separated by the stationary phase of the column through a variety of chemical and physical interactions.
Detectors
The detector converts information into an electrical signal by identifying the specific molecules that elute from the column. The type of analyte or sample being detected is also taken into consideration while choosing the detectors. It must be unaffected by modifications to the composition of the mobile phase.
Recorder
The electrical signal from the detector is passed through the recorder that results in the liquid chromatogram.
Applications of HPLC
HPLC is used for various purposes. Some of them are as follows:
- It is used for product purity and quality assurance for fine chemicals as well as industrial goods
- Separation and purification of biopolymers, such as nucleic acids or enzymes
- Measuring the amount of DEET in human urine also can be done by using HPLC.
- Finding endogenous neuropeptides in the extracellular fluid of the brain.
- Calculations of pharmaceutical product shelf lives.
- Detecting diphenhydramine in sediment sample analysis.
- Detecting anabolic steroids in urine, sweat, hair, and serum also.
- Measurement of psychotherapy medications in human plasma.
- Analysis of sugar in fruit juices.
- Ensuring the consistency and quality of soft drinks.
- Amino acids, which are ionic in nature, are best separated by ion exchange HPLC.
- It is also used for analysis of polycyclic aromatic hydrocarbons in vegetables and fruits.
Advantages of HPLC
- HPLC provides a quick, automated, and extremely accurate way to identify specific chemical components in a sample.
- HPLC is incredibly rapid and effective when compared to other chromatographic methods, like TLC.
- In general, when it comes to identifying and measuring chemical components, HPLC is flexible and incredibly accurate.
- It is very replicable.
- HPLC also can be used with mass spectroscopy (MS).
Disadvantages of HPLC
- HPLC can be expensive and also require the use of numerous expensive organics.
- The lack of a specific detector for HPLC, however, the UV-Vis detector only detects chromophoric compounds.
- Technical knowledge is needed to handle the instrument.
- The cleanliness of the sample, the mobile phase, and the effectiveness of the system all contribute to the reliability of the HPLC pump process.
Read also
References
- http://webhost.bridgew.edu/c2king/ch450/lec8_chrom3_hplc.pdf.
- https://polymer.ustc.edu.cn/_upload/article/files/23/46/8007190f4e29a2673dc0b5c292ba/P020110906263097048536.pdf.
- E. Heftmann 2004. Chromatography, Sixth Edition: Fundamentals and applications of chromatography and related differential migration methods – Part B: Applications
- https://www2.chemistry.msu.edu/courses/cem434/Swain_2015_Lecture%20Notes/HPLC%20Lecture1a.pdf.
- https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/high-performance-liquid-chromatography.
- https://www2.chemistry.msu.edu/courses/cem434/Swain_2015_Lecture%20Notes/HPLC%20Lecture1a.pdf.
- https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Instrumentation_and_Analysis/Chromatography/High_Performance_Liquid_Chromatography.
- https://microbenotes.com/high-performance-liquid-chromatography-hplc/.
