Helium is the second most abundant element in the universe. Helium is a noble gas and its symbol is He. The elements in Group 18 (VIIIA) of the periodic table are noble gases. It is colorless, odorless, and tasteless. It is monatomic and has an extremely low boiling point. The only element that cannot solidify by adequate cooling at normal atmospheric pressure is He. To solidify it, a pressure of 25 atmospheres at a temperature of 1 K (or -272 °C or -458 °F) must be applied. It is the only element in the periodic table that was discovered by an astronomer.
Interesting Science Videos
History
Helium was discovered in 1895 by Sir William Ramsay in London, and independently by Per Teodor Cleve and Nils Abraham Langlet in Uppsala, Sweden
Pierre. J. C. Jenssen
French astronomer made the initial discovery of helium in 1868 while researching the Sun’s chromosphere during a solar eclipse. P. Jenssen traveled to India in 1868 to examine the sun spectrum during a total eclipse and discovered a new yellow line, indicating the presence of a new element. Helium divided the light into its spectrum using a spectrometer, where each hue stands for a particular gaseous element. He noticed a fresh yellow glow and deduced that it meant a previously unknown element was present. A similar line was noted by Joseph Norman Lockyer, who called the new element helium after watching the sun through the pollution of London.
The Italian Luigi Palmieri discovered the same line in the spectrum of gases emitted by Vesuvius in 1882, as did the American William Hillebrand in 1889, when he collected the gas given off by the mineral uraninite (UO2) as it dissolves in acid. Per Teodor Cleve and Nils Abraham Langer, in Uppsala, Sweden, performed the experiment in 1895, confirming that it was helium and measuring its atomic weight.
Sir William Ramsay
Helium’s presence on Earth was established in 1895 by Sir William Ramsay. Helium, a byproduct of the radioactive elements’ natural disintegration, was discovered to have been released after heating the radioactive material cleveite. Helium was officially recognized as an element and given the Greek name helios, which means “sun,” by the chemist Norman Lockyer and Edward Frankland.
The discovery of helium in natural gas was initially not expected to have many applications. However, during World War I, scientists and military officials started to advocate for the use of helium in blimps. According to the ACS, helium blimps weren’t utilized much in World War I because of the high cost of production, but they were employed considerably more frequently during World War II when helium prices had decreased.
Occurrence and Abundance of Helium
- Helium is the second most abundant element in the universe after hydrogen, making up around 23% of the total mass of the universe.
- It is produced by nuclear fusion from hydrogen and is concentrated in stars.
- Even though the amount of helium on Earth is just 1 part in 200,000 (0.0005 percent) and only trace amounts are found in meteoric iron, radioactive minerals, and mineral springs, significant levels of helium are discovered as a component (up to 7.6 percent) in natural gases in the United States (especially in Texas, New Mexico, Kansas, Oklahoma, Arizona, and Utah).
- It is present in the Earth’s crust in only around 8 parts per billion, compared to the normal air’s 5 parts per million concentration.
Isotopes of Helium
Every helium atom has two protons in its nucleus, but like with all elements, there are different isotopes of He. The known He isotopes have masses ranging from three to eight and contain one to six neutrons.
Only two of these six isotopes, helium-3 ( 3He) and helium-4 (4He), are stable.
All the other isotopes are radioactive and degrade into other substances very quickly. The helium that exists on Earth has been produced via radioactive decay rather than being a primordial element. The nuclei of the isotope helium-4 are the alpha particles, which are released from the nuclei of heavier radioactive substances. Because Earth’s gravity is insufficient to stop helium’s slow escape into space, it does not build up in significant amounts in the atmosphere. The negative beta decay of the seldom 3H (tritium) isotope is what causes the small amount of 3He to exist on Earth .
The most common stable isotope is 4He, which outnumbers 3He atoms in atmospheric helium by about 700,000:1 and in some helium-bearing materials by about 7,000,000:1.
Physical Properties of Helium
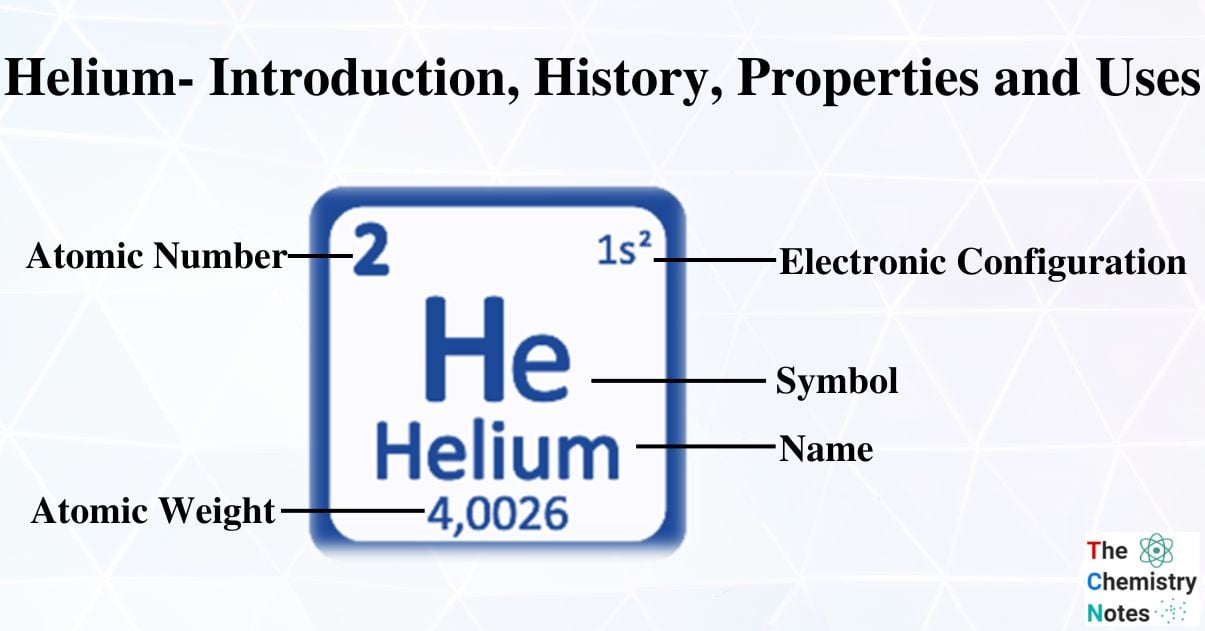
- He is a gaseous chemical element with the atomic number 2, the weight of 4,0026 g/mol, and the symbol He.
- It is one of the noble gases in group 0 of the periodic table. The second lightest element is this one.
- A colorless, odorless, bland, and non-toxic gas.
- Several natural gas sources in the United States are the primary source of He on the entire planet.
- It is the gas that is least soluble in water.
- It is the less reactive element and doesn’t combine with other elements to produce chemical compounds.
- Incredibly low helium vapor density and viscosity.
- The thermal conductivity of He is extremely high.
- Although He can be liquefied, it has the lowest condensation temperature of any known substance.
| Electronic Configuration | 1s2 |
| Block, Period and Group in periodic table | s-block, Group-18, Period-1 |
| Atomic Number | 2 |
| Atomic Weight | 4.00260 gmol-1 |
| State at 20 °C | Gas |
| Melting Point | 0.95 K (or -272.2oC) |
| Boiling Point | −268.928 °C, −452.07 °F, 4.222 K |
| Triple Point | 2.177 K; 5.043 kPa |
| Density | 0.000164 g/cm3 |
| Vander Waals Radius | 140 picometers |
| Covalent Radius | 0.37 Å |
| Energy of first ionisation | 2372.3 kJ mol -1 |
| Second ionization energy | 5250.5 kJ mol -1 |
| Enthalpy of Fusion | 0.0138 kJ mol -1 |
| Critical Temperature and Pressure | 5.195 K; 0.227 MPa |
| Crystal Structure | Hexagonal close-packed |
| Most Common Isotope | 2He4 |
| Discovery | Sir William Ramsay and independently by Per Teodor Cleve and Nils Abraham in 1895 |
| Named derived from | Greek word helios meaning sun |
Uses and Applications of Helium

- He is mostly used in meteorological balloons and altitude studies due to its low density. Also, other lighter-than-air vehicles like dirigibles require the use of helium (blimps).
- Also utilized to create an inert protective atmosphere for the production of fiber optics and semiconductors as well as for arc welding because of how little it reacts.
- It is also used to inflate car airbags after an accident since it diffuses quickly and can be utilized to detect leaks, such as those in automotive air conditioning systems. It can be used to search for leaks in long pipes where one or more leaks are suspected. The pipe has a detector held outside of it. The detector detects any He leakage from the system. You can find out if there is a leak, where it is, and how much of it there is. Because it doesn’t react with anything in the pipe, it is an excellent gas to employ for this application.
- The Large Hadron Collider (LHC) and the superconducting magnets in MRI scanners and NMR spectrometers both employ He as a cooling medium. Additionally, it was also applicable to cool the liquid oxygen and hydrogen that powered the Apollo spacecraft as well as the equipment on satellites.
- Additionally, preparing silicon and germanium crystals using helium. Superconductivity only happens at extremely low temperatures, which is an issue. Liquid helium is one method of reaching those temperatures. Liquid helium has several uses in cryogenics, magnetic resonance imaging (MRI), and superconducting magnets because of its low melting point.
Other Uses
- As a carrier gas, this element is also applicable in gas chromatography.
- For deep-sea divers and other people working in pressurized environments- an artificial atmosphere composed of 80% He and 20% oxygen.
- In supermarket checkout lanes, reading barcodes using helium-neon gas lasers. A helium-ion microscope, which provides superior picture resolution than a scanning electron microscope, is a new application for He.
- In low-temperature physics, He is utilized in the laboratory as a refrigerant or the coolest chemical.
Health Effects of Helium
Effects of exposure: Inhaling the material can cause absorption into the body. High voice on inhalation. Dizziness. Dullness. Headache. Suffocation. Frostbite can occur when liquid touches the skin.
Eyes: Frostbite when in contact with liquid.
Risk of Inhalation: If containment is lost, this gas can suffocate victims by reducing the oxygen concentration of the air in small spaces. Before entering the location, check the oxygen level. The more pure helium you inhale, the longer your body goes without vital oxygen. Pure helium inhalation can quickly result in asphyxiation and death. A gas or air embolism, which is a bubble that becomes caught in a blood vessel and blocks it, can also be brought on by inhaling helium from a pressurized tank.
Under normal circumstances, neutral helium is non-toxic, has no biological function, and is present in minute quantities in human blood.
Effects on Health Over Time: After being exposed to helium, the following chronic (long-term) health problems may manifest and may persist for months or years.
Toxicity, Safety, and Precautions Related to Helium
- Helium can affect you when inhaled.
Exposure to high levels can cause headache, dizziness, and lightheadedness. - Very high levels can cause passing out and death due to suffocation from lack of Oxygen
- Contact with liquid Helium can cause frostbite.
First Aid
Eye Contact: Immediately flush with large amounts of cool water for at least 15 minutes, lifting upper and lower lids. Remove contact lenses, if worn, while rinsing.
Skin Contact: Immerse affected part in warm water. Seek medical attention.
Inhalation: Remove the person from exposure. Begin rescue breathing (using universal precautions) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility.
Handling and Storage
- Keep in cool, well-ventilated areas in firmly covered containers, a place away from HEAT and SUN.
- Transport liquid helium and store it under positive pressure to prevent infiltration of air and other gases.
- Store at temperatures less than 125 degrees Fahrenheit.
- Ensure that ice does not form around the cylinder neck as the ice may cause the pressure valve to fail.
EMERGENCY NUMBERS
Poison Control: 1-800-222-1222
CHEMTREC: 1-800-424-9300
NJDEP Hotline: 1-877-927-6337
National Response Center: 1-800-424-8802
References
- Wiberg, Egon; Wiberg, Nils; Holleman, Arnold Frederick (2001). Inorganic chemistry. Academic Press. p. 240. ISBN 978-0123526519.
- https://www.rsc.org/periodic-table/element/2/helium
- Enghag, P. (2004). Encyclopedia of the elements.
- https://byjus.com/chemistry/helium/
- https://nj.gov/health/eoh/rtkweb/documents/
- https://www.lenntech.com/periodic/elements/he.htm
- https://www.britannica.com/science/ helium-chemical-element#ref283905
