Helium element, with the symbol He and atomic number 2, is the second most abundant chemical element in the universe. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas at the top of the periodic table of noble gases. It has the lowest boiling and melting points of any element. The low density is also responsible for the strange “squeaky voice” sensation while inhaling helium. The less thick the gas surrounding the vocal cords, the faster they vibrate, raising the pitch of the voice.
Its clinical value in inhalation therapy stems from its superior physical characteristics to ambient air. Helium element has a lower density than air and produces less resistance, allowing for better lung ventilation. It is also utilized as an analytical reagent in diagnostic/imaging procedures to detect the patient’s respiratory function.
Interesting Science Videos
Helium History
During a solar eclipse in 1868, French astronomer Jules Janssen spotted an unusual yellow line in the spectrum. Several months later, English chemists Joseph Norman Lockyer and Edward Franklin observed the same spectral line in sunlight and reached the same conclusion; the two proposed naming the element “Helium” after Helios, the Greek god of the sun, fitting for the first element discovered in space rather than on Earth. In 1895, Scottish chemist William Ramsay successfully isolated the element by processing a sample of the uranium ore cleveite, demonstrating its existence on Earth. Ramsay and Frederick Soddy discovered that helium is a byproduct of the spontaneous breakdown of radioactive compounds in 1903.
Helium was previously unknown to scientists due to its great chemical inertness, which made detection challenging using standard methods. This inertness is characteristic of “noble gases” (Group 18 on the periodic table, which includes neon and argon), whose exceptional stability and inability to react with other elements is owing to the completion of their outer valence shells.
Occurrence and Production of Helium Element
Although helium element is relatively sparse on Earth, it is the universe’s second most abundant element. Helium atoms account for approximately 11.3% of all atoms in the universe. Helium element traces can be found in the environment, and the air we breathe contains around 0.000524% helium.
The energy-producing fusion processes in stars produce large amounts. Helium was previously rarely used because it makes up only.0004% of the Earth’s atmosphere—that is, one helium molecule for every 200,000 air molecules, including oxygen, hydrogen, and nitrogen. The discovery of helium-rich wells in Texas, Russia, Poland, Algeria, China, and Canada, on the other hand, has made helium more available.
The primary sources of helium are natural gas reserves in wells in Texas, Oklahoma, and Kansas. Helium is recovered from natural gas, which contains up to 7% helium, by fractional distillation.
Radioactive decay produces helium in minerals. Helium is recovered from natural gas sources that contain up to 10% helium. The sole industrially available supply of helium is from these natural gas deposits. The world’s total helium resources are theoretically 25.2 billion cubic meters; the United States has 11.1 billion cubic meters. The extracted gas is pre-purified chemically with an alkaline wash to remove carbon dioxide and hydrogen sulfide. The remaining gas is cooled to -200°C when all materials are liquefied except helium gas.
Isotopes of Helium
Helium has eight known isotopes, however only helium-3 and helium-4 are stable. There is one He-3 atom for every million He-4 atoms in the Earth’s atmosphere. The presence of helium-3 on Earth is due to the negative beta decay of the uncommon hydrogen-3 isotope (tritium). Helium-4 is by far the most abundant of the stable isotopes: helium-4 atoms outweigh helium-3 atoms in atmospheric helium by 700,000:1 and by 7,000,000:1 in certain helium-bearing materials.
Helium element, on the other hand, is uncommon in that its isotope abundance fluctuates substantially depending on its source. The fraction of He-3 in the interstellar medium is roughly a hundred times higher. Isotope ratios in rocks from the Earth’s crust can vary by a factor of ten; this is utilized in geology to analyze the origin of such rocks. Every helium atom has two protons in its nucleus, however as with all elements, isotopes of helium exist.
Helium isotopes that contain one to six neutrons have mass values ranging from three to eight. Only the isotopes with mass numbers of three (helium-3, or 3He) and four (helium-4, or 4He) are stable; the others are radioactive, rapidly disintegrating into other substances. The helium found on Earth is not a primordial component, but rather the result of radioactive decay. Alpha particles are nuclei of the isotope helium-4 that are released from the nuclei of heavier radioactive substances. Because Earth’s gravity is insufficient to prevent its progressive escape into space, helium does not collect in huge quantities in the atmosphere.
Elemental Properties
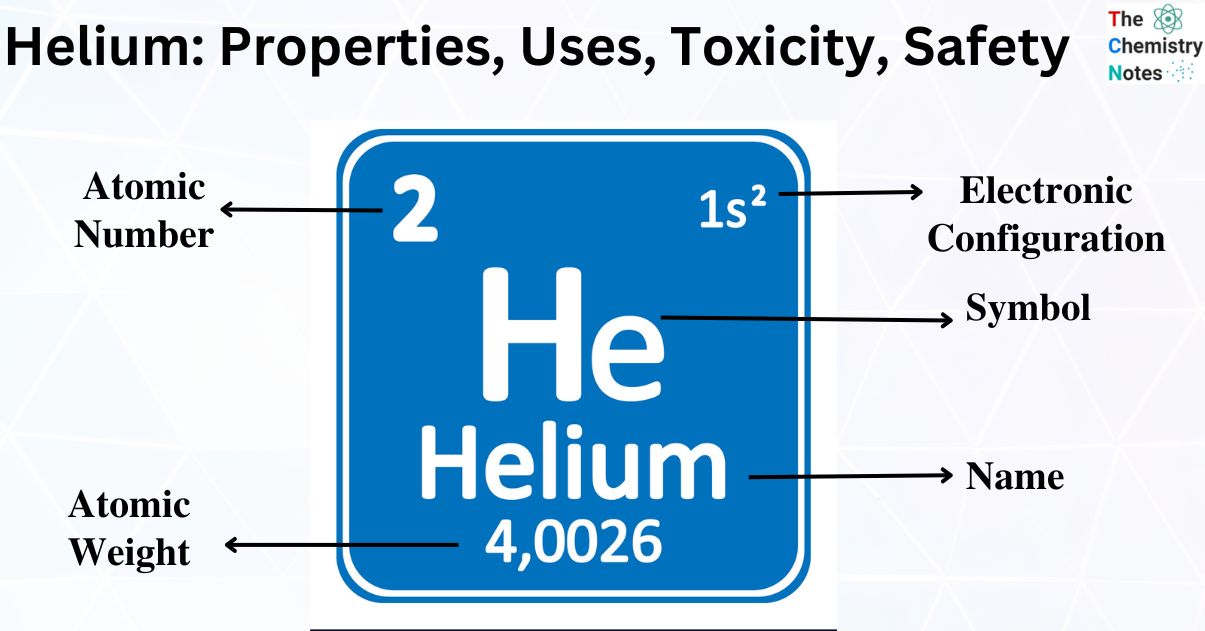
| Electronic Configuration | 1s2 |
| Atomic Number | 2 |
| Atomic Weight | 4.002602 |
| Group, Period, and Block | 18 (Noble Gases), 1, s-block |
| Covalent radius | 28 pm |
| Van der Waals radius | 140 pm |
| Electrons: | 2 |
| Protons: | 2 |
| Neutrons in the most abundant isotope: | 2 |
| Electron shells | 2 |
Physical Properties of Helium
- Helium is a gas that is colorless, odorless, and tasteless.
- It has the lowest boiling point (-268.9°C/-452.0°F) of any element.
- Helium has a freezing point of -272.2°C (-458.0°F).
- It remains liquid down to absolute zero at ordinary pressures but can be solidified by increasing the pressure.
- Below 2.18 K, the thermal conductivity of helium-4 exceeds that of copper by more than 1,000 times.
- When liquid helium is cooled to 3K, it transforms into a superfluid. It moves up and over the container’s walls, forming a drop on the container’s bottom. Superfluidity is an extremely unusual phenomenon that happens at extremely low temperatures. The important feature is zero viscosity.
- Helium II has a feature known as superfluidity, which means that its viscosity, or barrier to flow, is so low that it has not been measured. This liquid forms a thin film on the surface of any substance it comes into contact with, and this film flows without friction even against gravity.
- At temperatures below roughly 0.8 K (-272.4 °C or -458.2 °F), a liquid mixture of the two isotopes helium-3 and helium-4 separates into two layers.
- The dissolution of helium-3 in helium-4 produces a cooling effect, which has been employed in the design of cryostats (devices for producing very low temperatures) capable of reaching and maintaining temperatures as low as 0.01 K (-273.14 °C, or -459.65 °F) for days.
| Helium (He) | Physical Properties |
| Melting Point | 0.95 K (or -272.2oC) |
| Boiling Point | 4.222 K (or -268.928oC) |
| Density | 0.1786 g/L at STP; 0.145 g.cm-3 at its melting point |
| Critical Temperature and Pressure | 5.195 K; 0.227 MPa |
| Triple Point | 2.177 K; 5.043 kPa |
| Appearance (at STP) | Colorless gas |
Chemical Properties of Helium
When an element engages in numerous chemical processes and interacts with its environment, its chemical characteristics can be seen. Helium element is inert and thus does not participate in many chemical reactions. Helium element has the electrical configuration 1s2. It has a refractive index closer to one and is generally known as the least soluble gas in water. Helium element found outside the earth’s atmosphere occurs in the plasma state, and the existence of free electrons in the plasma state results in exceptionally high electric conductivity.
| Helium (He) | Chemical Properties |
| Electron Configuration | 1s2 |
| First Ionization Energy | 2372.3 kilojoules per mole |
| Second Ionization Energy | 5250.5 kilojoules per mole |
| Van der Waals Radius | 140 picometers |
| Enthalpy of Fusion | 0.0138 kilojoules/mole |
Uses and Applications of Helium
Helium has numerous distinct qualities, including a low boiling point, low density, low solubility, high thermal conductivity, and inertness, making it suitable for usage in a wide range of applications.
- Helium is mostly used in altitude research and meteorological balloons.
- In autogenous welding, it is used as an inert protective gas.
- It is the only cooler capable of dropping temperatures below 15K (-434ºF).
- To keep divers and others who operate in pressurised situations safe, an artificial atmosphere is created using 20% oxygen and 80% helium. Analox specializes in the ability to consistently monitor this artificial environment.
- Helium element is also employed in the manufacture of germanium and silicon crystals.
- Helium element is employed in pipeline leak detection because of its ability to permeate through solids considerably faster than air.
- This element is also employed as a carrier gas in gas chromatography.
- Liquid helium has several applications in cryogenics, magnetic resonance imaging (MRI), and superconducting magnets due to its low melting point.
- The principal application of ultralow temperature is the formation of the superconducting state, in which the resistance to the flow of electricity is almost nil.
- Additional uses include pressurizing gas in liquid rocket propellants, helium-oxygen mixes for dives, and the working fluid in gas-cooled nuclear reactors
- Because helium has a low boiling point, it is utilized as a coolant in many technological marvels. Liquid helium is used to cool the superconducting magnetic coils in CERN’s LHC particle accelerator.
Read Also: Chlorine Element
Health and Environmental Effects of Helium
At standard conditions, neutral helium is non-toxic, serves no biological role, and is found in trace amounts in human blood.
- The speed of sound in helium is roughly three times that of air. Because the natural resonance frequency of a gas-filled cavity is proportional to the speed of sound in the gas, inhaling helium causes an increase in the resonant frequencies of the vocal tract, which serves as an amplifier of vocal sound. The high-frequency-preferred amplification, on the other hand, alters the timbre of the amplified sound, resulting in a reedy, duck-like vocal tone. Inhaling a dense gas such as sulfur hexafluoride or xenon has the reverse effect of reducing resonance frequencies.
- There is always the possibility of other impacts, such as: nausea, lightheadedness, and fainting.
- The majority of serious health problems and deaths caused by helium inhalation are the result of inhaling helium from a pressurized tank. These are the identical tanks that are used to fill helium balloons at parties and in party supply stores.
- The purer helium you breathe in, the longer your body goes without oxygen. Inhaling pure helium might result in death by asphyxiation in minutes.
- Inhaling helium from a pressurized tank can also result in a gas or air embolism, which is a trapped bubble in a blood vessel that blocks it. Blood vessels can rupture and cause hemorrhage.
- The second most frequent element in the universe is helium. On Earth, nevertheless, it is uncommon. It is the world’s only nonrenewable resource. Hence, when using helium, people must use extreme caution. In reality, it takes the Earth hundreds of years—or many millennia—to manufacture the element once more.
- Helium is a light, non-reactive element. It can so readily elude the Earth. In other words, it actually vanishes from Earth and travels directly into space. These characteristics prevent it from being recycled. Also, the substance does not cause any pollution. In actuality, it has no harmful consequences on the environment.
Toxicity, Safety, and Protection
Helium is not poisonous. There will be no negative environmental repercussions. Helium does not contain any ozone-depleting compounds of Class I or Class II. DOT does not designate helium as a marine contaminant.
Helium is a harmless, odorless, colorless, and nonflammable gas that is stored under high pressure in cylinders. When concentrations are high enough to lower oxygen levels below 19.5%, it can quickly suffocate a person. It can accumulate in high places or along ceilings since it is lighter than air. The use of Self-Contained Breathing Apparatus (SCBA) by rescue personnel is possible.
- Those who are deficient in oxygen should be transported to fresh air. If the victim is not breathing, artificial respiration should be administered. If breathing becomes difficult, oxygen should be administered. Get medical assistance as soon as possible.
- Helium is not flammable and cannot be burned. Extinguish the fire with a suitable extinguishing medium.
- Helium is a straightforward asphyxiant. Remove helium cylinders from the fire area if possible, or cool them with water. Rescue workers may need self-contained breathing apparatus. When exposed to strong heat or flame, the cylinder will rapidly vent and/or rupture violently. When exposed to high temperatures, most cylinders are intended to evacuate their contents. Heat can cause pressure to build up in a container, causing it to rupture if pressure relief systems fail to function.
- Remove all personnel from the affected area. Promote airflow to the releasing area and keep an eye on the oxygen level. Use adequate safety equipment (SCBA). If the leak is coming from the container or its valve, dial the Air Products emergency hotline. If there is a leak in the user’s system, close the cylinder valve and vent the pressure before trying repairs.
Storage and Handling Care
- Cylinders should be stored upright in a well-ventilated, safe, and weather-protected place. Temperatures in the storage facility should not exceed 125 °F (52 °C), and the area should be devoid of combustible objects. Storage should be kept away from high-traffic areas and emergency exits.
- Avoid regions that include salt or other caustic materials. Valve protection covers and valve outlet seals should be left on cylinders that are not in use. Separate the full and empty cylinders.
- Avoid having too much inventory and storing it for too long. Make use of a first-in, first-out mechanism. Maintain accurate inventory records.
- Do not drag, roll, or move the cylinder. Employ a hand truck made specifically for cylinder movement. Never try to raise a cylinder by the cap. While in use, keep cylinders secure at all times.
- To safely release gas from the cylinder, use a pressure-lowering regulator or a separate control valve.
- To prevent reverse flow into the cylinder, use a check valve. To raise pressure or discharge rate, do not overheat the cylinder. If the user has any difficulties using the cylinder valve, stop using it and contact the provider. Never place an object (e.g., a wrench, screwdriver, pry bar, etc.) into the openings of valve caps. This could damage the valve, resulting in a leak. To remove over-tightened or corroded caps, use an adjustable strap wrench.
- Helium is compatible with all basic building materials. While selecting materials and designing systems, pressure requirements should be taken into account.
EMERGENCY TELEPHONE NUMBERS
800 – 523 – 9374 Continental U.S., Canada and Puerto Rico
610 – 481 – 7711 other locations
Fun Facts of Helium
- Under normal atmospheric pressure, helium is the only element that cannot be solidified using proper cooling processes. The zero-point energy of the system is too high to allow for freezing.
- During the Cold War and Space Race, rocket fuel used Helium as a coolant.
- Expansion cooling can be used to liquefy helium.
- High-pressure heating can introduce helium into the hollow carbon cage of fullerenes.
- Helium may be extracted from significant amounts of natural gas using fractional distillation for large-scale industrial purposes.
- Helium derived from natural gas sources in Kansas, Oklahoma, and Texas’ Panhandle Field has been depleted and sold since 2005. It is predicted to be exhausted completely by 2021.
- Saturn has a helium content of 3%.
- The United States produces around 90% of the world’s helium.
- During World War Two, the need for helium surged. It was employed by lighter-than-air aircraft.
- A helium mass spectrometer was used to detect and pinpoint minor leaks during the Manhattan Project, which produced the first nuclear weapons.
- Because helium is nonrenewable, the Helium Act of 1925 prohibited its export.
Find out more interesting Information related to Helium.
References
- Isotope masses from Ame2003 Atomic Mass Evaluation by G. Audi, A.H. Wapstra, C. Thibault, J. Blachot and O. Bersillon in Nuclear Physics A729 (2003).
- Isotopic compositions and standard atomic masses from Atomic weights of the elements. Review 2000 (IUPAC Technical Report). Pure Appl. Chem. Vol. 75, No. 6, pp. 683-800, (2003) and Atomic Weights Revised (2005).
- Meija, J.; et al. (2016). “Atomic weights of the elements 2013 (IUPAC Technical Report)”. Pure and Applied Chemistry. 88 (3): 265–91. doi:10.1515/pac-2015-0305
- Shuen-Chen Hwang, Robert D. Lein, Daniel A. Morgan (2005). “Noble Gases”. Kirk Othmer Encyclopedia of Chemical Technology. Wiley. pp. 343–383. doi:10.1002/0471238961.0701190508230114.a01.
- Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- https://www.britannica.com/science/helium-chemical-element#ref283903
- https://www.lenntech.com/periodic/elements/he.html
- https://byjus.com/chemistry/helium/
- https://chemistrytalk.org/helium-element/


This article provides a comprehensive overview of helium, covering its history, properties, uses, and safety considerations. It’s fascinating to learn about the various applications of helium, from altitude research to cryogenics and beyond. The safety precautions outlined here are crucial, especially regarding its potential hazards when inhaled improperly. Overall, this is a valuable resource for anyone interested in understanding more about this unique element.