A heat engine draws heat from a source and uses it to create mechanical work for a variety of purposes. For instance, a steam engine on an antique train can generate the necessary work to propel the vehicle. The role of automobile engines is crucial for transportation in today’s technological world. Engines that use gasoline or diesel as input and rotate the wheels to move the vehicle are found in motorbikes and automobiles. The majority of these car engines are only 40% efficient. Engine efficiency is severely constrained by the second law of thermodynamics. Consequently, it is crucial to comprehend heat engines.
What is Heat Engine?
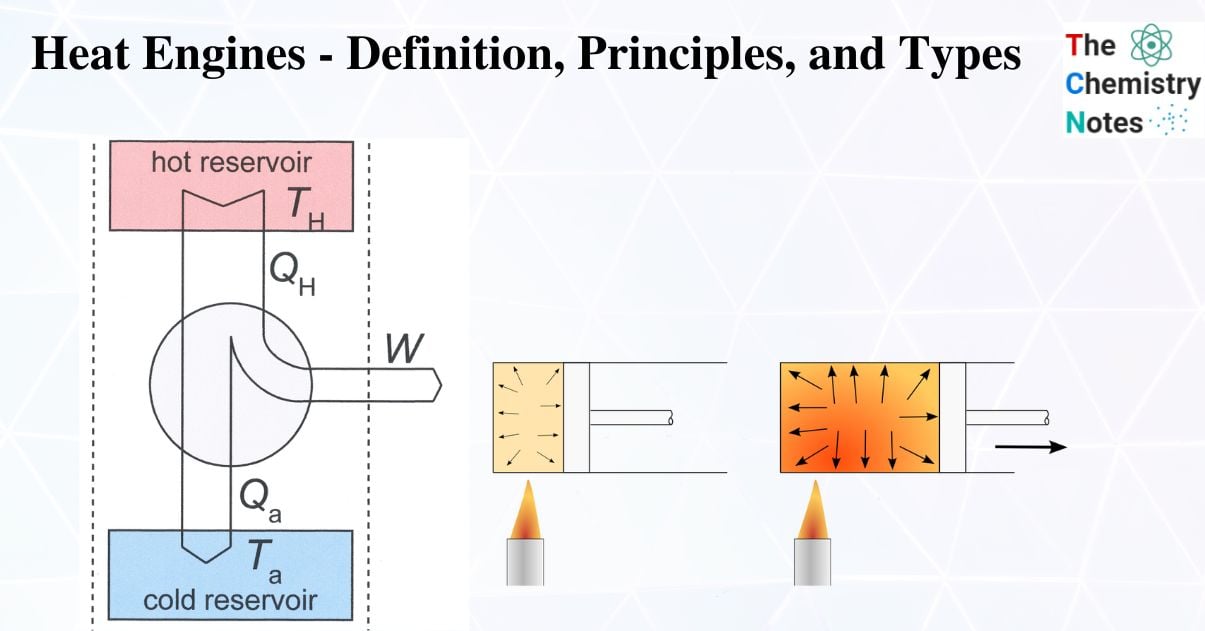
A heat engine is a system that converts heat into work by using heat from a reservoir (hot body) to perform some task.
Some heat is discharged to the sink (cold body). There will also be some waste in the form of heat in this system. There will also be some waste in the form of heat in this system.
A heat engine uses heat to generate power. It draws heat from a reservoir, uses that heat to do work, such as move a piston or lift weights, and then releases that heat energy into the sink.
Petrol Engine and Diesel Engine are the examples of Heat Engines.
A heat engine is a system in thermodynamics and engineering that converts heat to mechanical energy, which can then be used to perform mechanical work. It accomplishes this by lowering the temperature of a working substance from one that is higher.
Thermal energy produced by a heat source raises the temperature of the working substance. The working substance transfers heat to the colder sink until it reaches a lower temperature state while producing work in the engine’s working body. The working material can be any system with a heat capacity greater than zero, but it is typically a gas or liquid.
- A system with a large thermal energy capacity known as a thermal reservoir can supply or absorb heat in finite amounts while maintaining a constant temperature.
- A reservoir that stores energy as heat is referred to as a source, and one that absorbs energy as heat is referred to as a sink. For instance, atmospheric air serves as both a source and a sink for heat pumps and air conditioners.
Principle of Heat Engine
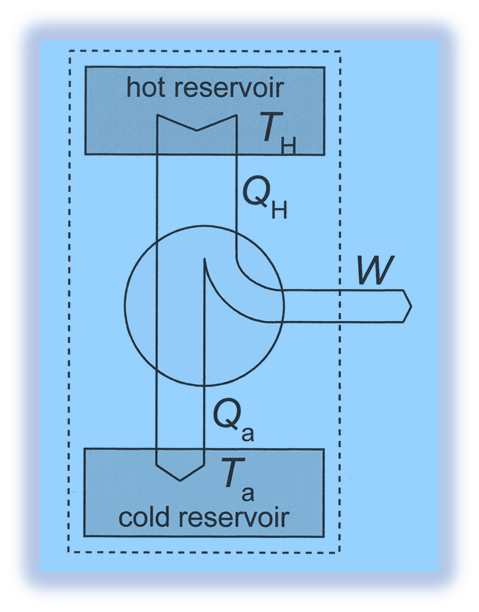
- When heat is applied to the working material from a high-temperature source, some of that heat is converted into work while the remainder is retained in the heat sink at a low temperature. The heat engine operates on a similar principle.
- The heat sink temperature should not be higher than the source temperature from which the engine receives its heat. This means that the engine receives heat from a source with a higher temperature, converts some of it into work, rejects the remainder to a heat sink with a lower temperature, and then returns to its initial state. This cycle change will allow the engine to produce work continuously.
- During the course of their operation, heat engines take in heat from a high-temperature source, convert some of this heat into work, and then reject the remaining waste heat to a low-temperature sink.
A furnace serves as the energy source and transfers heat (Qin) to the steam in the boiler.
Steam flowing through the turbine causes it to produce work (Wout)
The waste heat (Qout) from steam is transferred by a condenser to an energy sink, such as the atmosphere.
Water is returned to the boiler from the condenser using a pump. Compressing water to boiler pressure requires work (Win).
The difference between the work output and the work input is the power plant’s net work output.
Wnet (output) = Wout – Win
The cycle’s energy balance reveals that the net work output is.
Wnet (output)= Qin – Qout
Types of Heat Engine
According to the theory that underpins how they operate, heat engines have been categorized.
The following are illustrations of various kinds of heat engines that can be found in thermodynamics:
- Internal Combustion Engine
- External combustion Engine
Internal Combustion Engine
A heat engine known as an internal combustion engine (ICE or IC engine) burns fuel in a combustion chamber that is a part of the working fluid flow circuit along with an oxidizer (typically air). An internal combustion engine component is directly impacted by the expansion of the high-temperature, high-pressure gases created by combustion. Typically, the force’s targets are nozzles, turbine blades, pistons, or rotorszThis force transforms chemical energy into usable kinetic energy, which is then used to propel, move, or power whatever the engine is connected to. The external combustion engine has been replaced by this for applications where engine weight or size are critical.
- The very first step of the process, drawing a fuel-air mixture into the heat engine, is carried out by the piston. In the cylinders of the heat engines, these pistons move vertically. A stroke is a single upward or downward movement of the piston inside the cylinder.
- The mixture is intensely adiabatically compressed in the second step.
- The third stage involves the ignition process, which significantly increases the temperature and pressure.
- As the process progresses, Piston clears the gases once more as its temperature increases.
External Combustion Engine
The fuel burns externally and away from the primary engine, which generates force and motion, in these heat engines. An illustration of an external combustion engine is the steam engine. Electricity and internal combustion engines have rendered steam engine locomotives largely obsolete.
For instance, steam trains are now only used as historical transportation or as tourist attractions. On an industrial scale, steam is still widely used to generate electricity. In a boiler (hot reservoir), water is heated from a heat source until it turns into steam, which is used to spin a turbine as it rises. This is an illustration of a heat engine, which transforms thermal energy into mechanical work. The electric generator that is powered by the rotating turbine then generates electricity for our use.
- Similar to an internal combustion engine, a fuel-air mixture must be drawn into the heat engine by the piston before it can be sent to the first cylinder.
- High temperature and increased pressure are produced by heating the gas in the first cylinder. The Piston gathers it once more and converts it into work. After that, it is transferred to another cylinder.
- The gas differs from the previous level and is also simple.
- Finally, the compressed gas is transferred back to the original chamber, where the Piston collects it and uses it to produce work.
Internal combustion engines are more efficient than external combustion engines when comparing the two types of heat engines. This is because, unlike in an external combustion engine, no energy is lost when heat is transferred from the boiler to the cylinder in an internal combustion engine.
How a Heat Engine Works
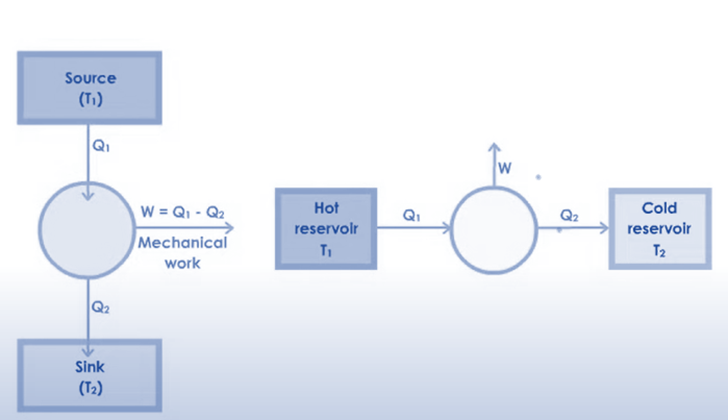
As we saw earlier, a heat engine basically consists of an engine, a cold sink, and a heat reservoir. The engine, where the piston is moved, receives the heat that we generate through internal or external combustion. The work is completed when the power generated is supplied to the engine-connected machine. The sink receives extra heat, keeping the reservoir and sink at the same temperature.
Similar to car engines, heat engines operated in cycles. In one part of the cycle, they will add energy in the form of heat, and in another part of the cycle, they will use this energy to carry out useful work.
Parts of Heat Engine
A heat engine has three crucial components, and they are as follows:
Source: There must be a heat source with infinite thermal capacity that is maintained at a constant high temperature, such that the temperature of the source is unaffected by the addition or removal of heat.
The working substance must be some sort of material that either accepts or rejects heat into the sink. This is the substance that is active.
Sink: There must be a thermal sink with a finite thermal capacity that is maintained at a constant high temperature so that no heat is taken from or given to it and the temperature remains constant.
Heat Engine Efficiency
The efficiency is the percentage of heat input that changes to work at high temperatures. According to the second law of thermodynamics, no engine is completely efficient.
Efficiency (η) = Work done / Heat input
We know that,
Work done (W) = Q1 – Q2
Heat input = Q1
Then,
Efficiency (η) = W / Q1
= (Q1 – Q2) / Q1
=1 – (Q2 / Q1)
Therefore, if Q2 = 0, efficiency will be 100%; however, this is not actually possible because there will be some energy loss in the system. Therefore, the efficiency of every engine has a limit. A reversible engine, like the Carnot heat engine, operates at its highest efficiency.
Ideal Heat Engine
A heat engine that is designed to only take heat from a high-temperature environment and convert it to work is not possible to create.
In other words, it is impossible to build a heat engine that doesn’t release heat into the atmosphere.
Alternatively, a heat engine that operates at 1.00, or 100% efficiency, cannot be built.
PV-diagram of Heat Engine
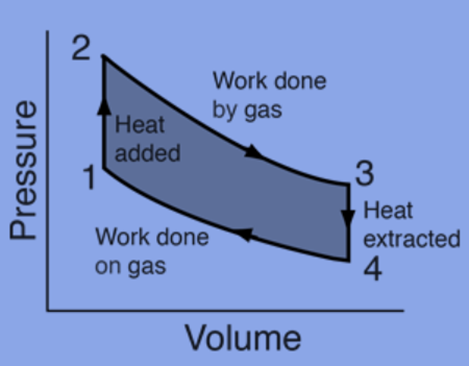
Heat engines, such as those found in automobiles, work in a cyclical fashion, adding energy in the form of heat in one phase and using that energy to perform useful work in the next.
The fundamental tool for studying heat engines that use gas as the working substance is a pressure-volume (PV) diagram.
A closed-loop PV diagram will be used for a cyclic heat engine. The amount of work completed during the cycle is represented by the area of the loop.
- If the source is hot, the fluid isothermally transforms from liquid to vapour. With increasing volume and constant pressure, this vaporization process takes place.
- The gas expands adiabatically and reversibly at the turbine end, and it does so by following the equation of state for an adiabatic and reversible process.
- If the source is at a low temperature, the fluid isothermally transforms from a gas to a liquid. Constant pressure and decreasing volume are the conditions for this condensation process.
- By increasing pressure, the liquid is reversibly and adiabatically compressed at the compressor end.
References
- Fundamentals of Classical Thermodynamics, 3rd ed. p. 159, (1985) by G. J. Van Wylen and R. E. Sonntag
- https://byjus.com/jee/heat-engine/
- https://www.vedantu.com/physics/heat-engine
- https://www.khanacademy.org/science/physics/thermodynamics/laws-of-thermodynamics/a/what-are-pv-diagrams
- Kroemer, Herbert; Kittel, Charles (1980). Thermal Physics (2nd ed.). W. H. Freeman Company.
- https://collegedunia.com/exams/heat-engines-physics-articleid-813
