The Grignard reagent is an organomagnesium compound with the chemical formula R – MgX, where R represents an alkyl or aryl group and X represents a halogen. In general, they are synthesized by reacting an alkyl or aryl halide with magnesium in the presence of dry ether.
In 1900, the French chemist Francois Auguste Victor Grignard discovered these reagents. Grignard was awarded the Nobel Prize in Chemistry in 1912 for his discovery.
Grignard discovered that an exothermic reaction occurs when magnesium turning is stirred with an ether of THF (tetrahydrofuran) solution of an alkyl or aryl halide. Magnesium, which is insoluble in ether, disappears as it reacts with the halide to form an ether-soluble Grignard reagent solution.
R – X + Mg → R – MgX (In presence of dry ether)
Ar – X +Mg → Ar – MgX
During the preparation of the Grignard reagent C – X, the bond is broken, and both alkyl or aryl group and halogen become bonded to Mg. Organic halides such as aryl or alkyl chloride, bromide, and iodide are used in these reactions. Because aryl and alkyl fluorides are not very reactive, they have rarely used. The order of reactivity of halides with Mg is:
RI > RBr > RCl
CH3I + Mg → CH3 – MgI (95 %) (In presence of ether or THF, at 35oC)
Solvent used for the preparation of the Grignard reagent
Diethyl ether is commonly used as a solvent. Ether should be dry, and free of traces of water or alcohol. Ether plays an important role in the preparation of Grignard reagents. The unshared electron pairs on the ether oxygen help to stabilize the magnesium through coordination. Ether acts as the Lewis base and stabilizes a the reagent.
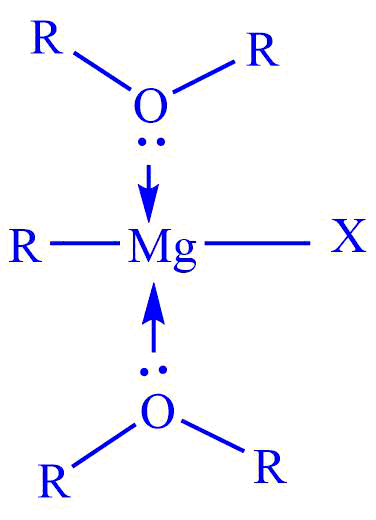
The complex formation with the molecules of the ether is an important factor in the formation and stability of Grignard reagents.
Water and air are very damaging to this synthesis and can quickly destroy the Grignard reagent that is being formed via protonolysis or reagent oxidation. As a result, the process must be carried out in an air-free environment.
Nature of Grignard reagent
The carbon-magnesium bond is covalent but highly polar with carbon-pulling electrons from electron-positive Mg, the Mg-X bond is essentially ionic.
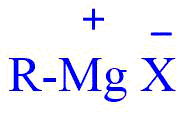
Since magnesium becomes bonded to the same carbon that previously held halogen, the alkyl group remains intact during the preparation of the reagent. For example, In presence of ether, n-propyl chloride yields n-propyl magnesium chloride.
CH3 – CH2 – CH2Cl + Mg → CH3 – CH2 – CH2MgCl
The Grignard reagents are highly reactive. They usually react as if the alkyl or aryl group is negatively charged and the Mg atom is positively charged.
Carbanions are strong bases, so the Grignard reagent reacts vigorously with weak acids like water or with other compounds with O – H, S – H, or N – H bonds. So, for the preparation of the Grignard reagent water and air are very harmful and ether used as a solvent that must be free of water or alcohol.
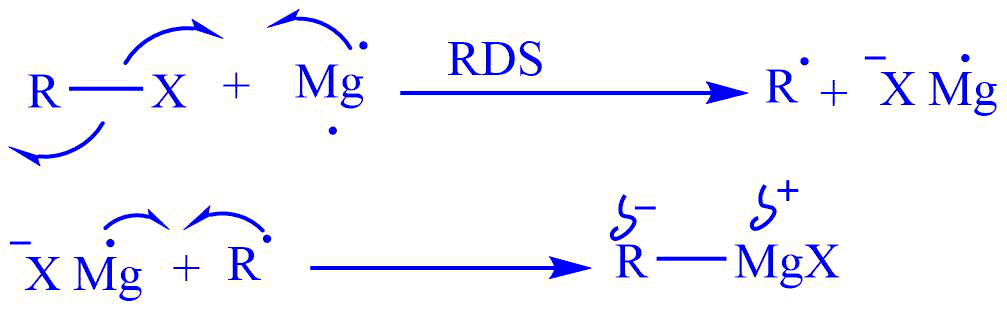
Mechanism of formation of the Grignard reagent
The formation of the Grignard reagent involves free radical intermediates. However, it does not involve a radical chain mechanism. It is non – radical mechanism.
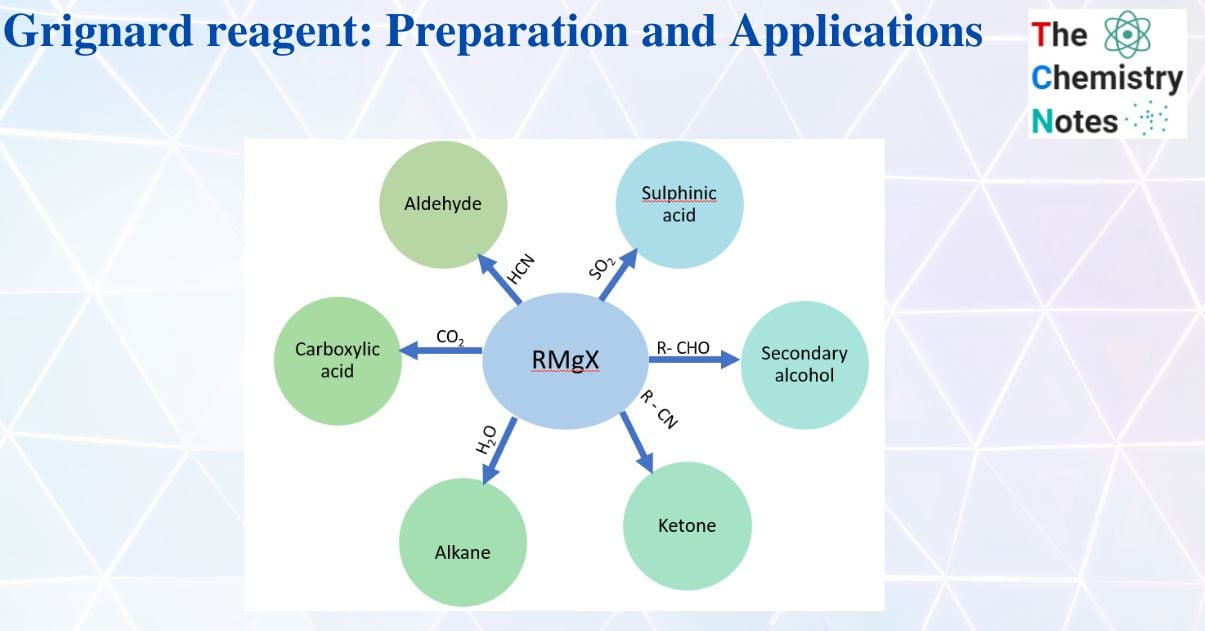
Application of Grignard reagents
1. Alkane formation
The Grignard reagent reacts with water, alcohol acid, and amine to give corresponding hydrocarbons.
E.g., Propylmagnesiumbromide produces propane gas when treated with water.
CH3 – CH2 – CH2 Mg Br + H2O → CH3 – CH2 – CH3 + Mg (OH) Br
When the Grignard reagent is treated with an alkyl halide, a coupling reaction occurs to give alkane. This reaction is catalyzed by the cuprous ions.
E.g., Methyl magnesium bromide reacts with methyl bromide to produce ethane.
CH3 – MgBr + CH3Br → CH3 – CH3 + MgBr2
2. Preparation of alcohol
The Grignard reagent reacts with carbonyl compounds in presence of dry ether to give an additional product which on acidic hydrolysis gives corresponding alcohol. This is a nucleophilic addition reaction. Depending on the nature of carbonyl compounds used, alcohols may be 1o, 2o, and 3o.
I. Reaction with aldehyde
Addition of Grignard reagents to formaldehyde yields primary alcohols. While the addition of Grignard reagent with aldehyde other than formaldehyde gives secondary alcohol.
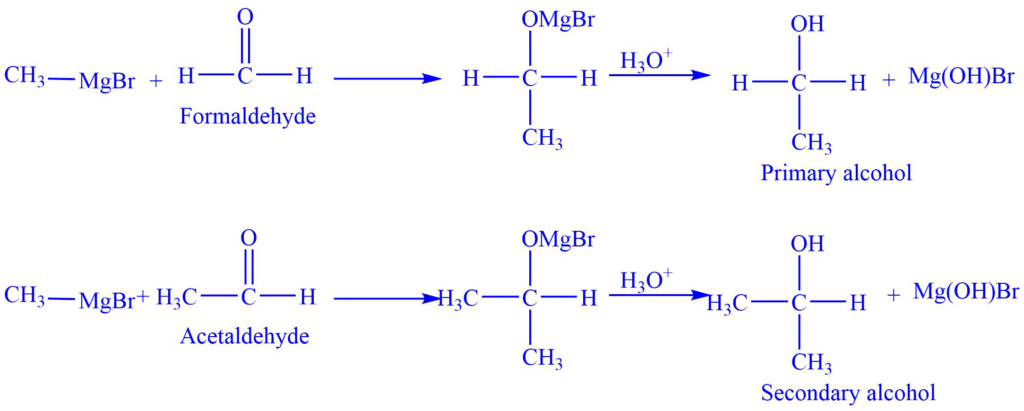
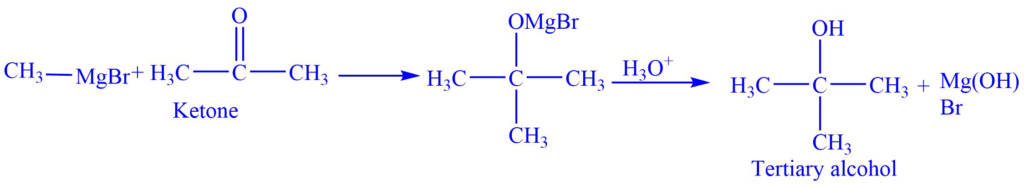
II. Reaction with ketone
The reaction of ketone gives tertiary alcohol.
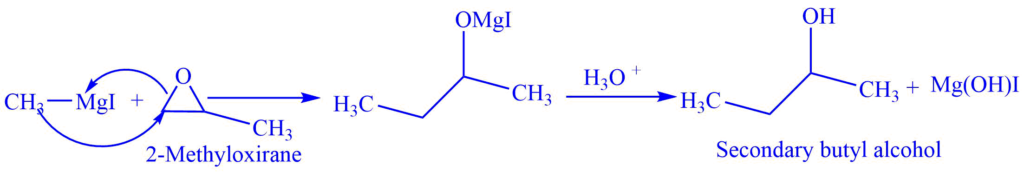
II. With oxirane
Oxiranes (epoxides) reacts with the Grignard reagent to produce alcohol. E.g., When 2-methyloxirane reacts with methylmagnesium iodide, secondary butyl alcohol is formed. The alkyl group of Grignard reagent substitutes the less substituted carbon of oxirane.
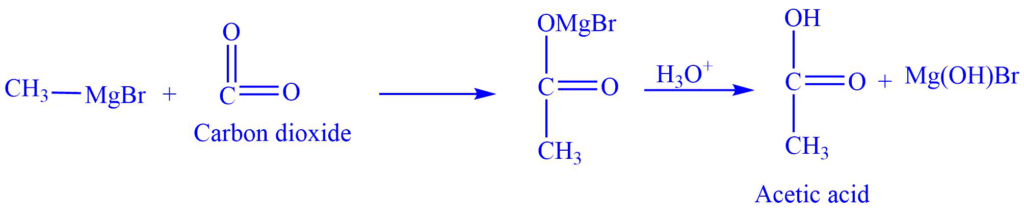
3. Formation of acid
a. The Grignard reagent reacts with carbon dioxide to give carboxylic acid.
For example., methylmagnesium bromide reacts with carbon dioxide to give acetic acid.
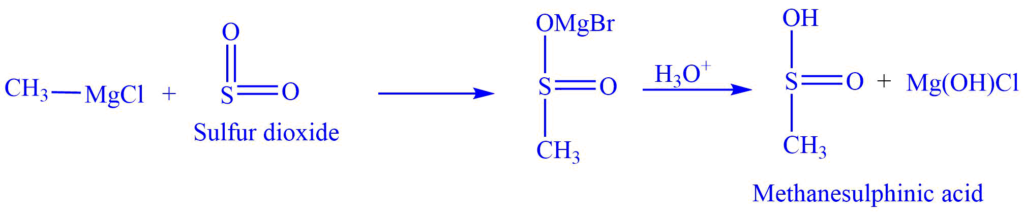
b. Reaction of the Grignard reagent with Sulfur dioxide SO2 to produce alkane sulphinic acid.
Methanesulphinic acid, for example, is produced when methylmagnesium chloride reacts with sulfur dioxide, SO2.
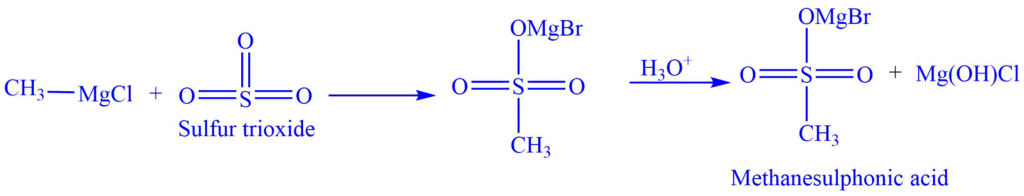
c. When the Grignard reagent reacts with sulfur trioxide (SO3), alkane sulphonic acid is formed.
E.g., Methanesulphonic acid is formed when methylmagnesium chloride reacts with sulfur trioxide (SO3).
4. Preparation of ketone
Nitriles, in reaction with the Grignard reagent, produce Ketone. However, when the Grignard reagent is added with hydrogen cyanide, the aldehyde is obtained.
E.g., Acetonitrile, for example, produces acetone when reacted with methyl magnesium iodide.
CH3 – MgI + CH3 – CN → (CH3)2 C = N -MgI + H2O → CH3 – CO – CH3 + Mg (OH) I + NH3
CH3 – MgI + H – CN → CH3CH = N -MgI + H2O → CH3 – CO – H + Mg (OH) I + NH3
5. Other preparative reactions
1. Reaction of Chloramine, and NH2Cl with a Grignard reagent to produce amines.
CH3 – MgX + Cl – NH2 → CH3 – NH2 + MgXCl
2. Nitriles can be prepared by the reaction of Grignard reagents with cyanogen or cyanogen chloride.
CH3 – MgX + NCCl (cyanogen chloride) → CH3 – CN + MgX(CN)
3. Grignard reagents can be used to prepare alkyl iodides. Iodine is added to the alkyl magnesium chlorides or bromides to produce the corresponding alkyl iodides.
CH3 – MgBr + I2 → CH3 – I+ MgICl
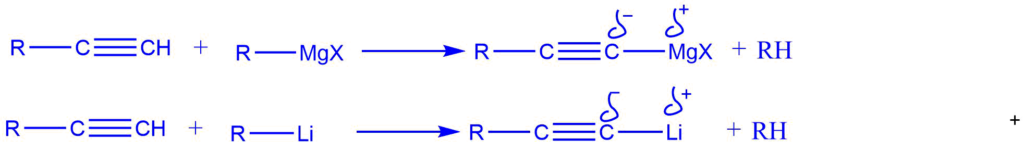
4. Grignard reagents and organolithium compounds react with the terminal hydrogen atoms of alkynes to give alkyl magnesium halides and alkynyl lithiums. These reactions are acid–base reactions.
Summery chart of the Grignard regent’s use in organic synthesis
| S.N | Reaction of the Grignard reagent with | Product |
| 1. | Formaldehyde (-HCHO) | Primary alcohol |
| 2. | Aldehyde (R- CHO) | Secondary alcohol |
| 3. | Ketone (R- CO – R) | Tertiary alcohol |
| 4. | Ester (R- CO – ORI) | Tertiary alcohol |
| 5. | Acid halide (R – COX) | Tertiary alcohol |
| 6. | Nitriles (R – CN) | Ketone |
| 7. | Oxiranes (epoxides) | Alcohol |
| 8. | Cynogen | Nitrile |
| 9. | Sulphur | Thiol |
| 10. | Water ((H2O) | Alkane |
| 11. | Carbon dioxide (CO2) | Carboxylic acid |
| 12. | Sulfur dioxide (SO2) | Sulphinic acid |
| 13. | Sulfur trioxide (SO3) | Sulphonic acid |
| 14. | Hydrogen cyanide (HCN) | Aldehyde |
| 15. | Alkylhalide ( R -X) | Alkane |
References
- Smith M. B. & March J. (2007). March’s advanced organic chemistry : reactions mechanisms and structure (6. ed.). Wiley-Interscience.
- Bahl A. & Bahl B. S. (2006). A textbook of organic chemistry (for b. sc. students) (18th rev. & enlarged ed. 1st multicolour illustrative). S. Chand.
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Aldehydes_and_Ketones/Synthesis_of_Aldehydes_and_Ketones/Grignard_Reagents
- Seyferth, D. (2009). The grignard reagents. Organometallics, 28(6), 1598-1605.
- https://www.masterorganicchemistry.com/2011/10/14/reagent-friday-grignard-reagents/
.
