Gravimetric analysis is an analytical technique used for the quantitative determination of an analyte based on the mass of solid.The element to be identified is precipitated from a solution using this method of analysis by the addition of a suitable precipitating agent. The precipitate should either have a known composition or, through heating, should be changed into another compound with a known composition.
For example, to determine the sulphate ions (SO4 2- ) contained in ammonium sulphate (NH4)2SO4 solution, the solution is trated with barium chloride (BaCl2) first. When all sulphate ions have precipitated as barium sulohate (BaSO4), the precipitate of barium sulohate is filtered, washed, dried, ignited, and finally weighed. By knowing the weight of the precipitate, BaSO4, the amount of sulphate ion present in the given volume of ammonium sulphate can be determined using a suitable stiochiometric relationship.
Principle of Gravimetric Analysis
Gravimetric Analysis is based on the principle the mass of the ion present in the pure compound can be determined by estimating the mass percentage of the same ion in the known quantity of an impure compound.
Steps involved in Gravimetric analysis
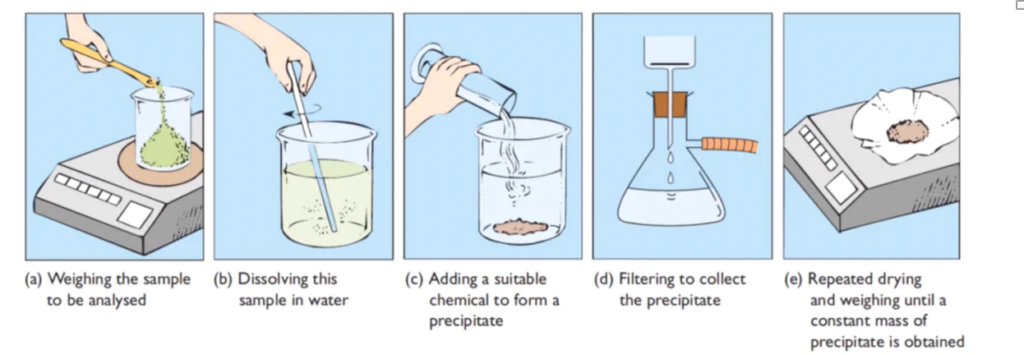
Image source: https://scienceready.com.au/pages/gravimetric-analysis
i) Preparation of solution
The specified sample was weighed in a watch glass and transferred to a beaker. If the salt sample is water soluble, it is dissolved in water to produce a sample solution. Salts that are insoluble in water dissolve in dilute acids. More water may be added to provide the appropriate volume of the solution once the sample has completely dissolved.
II) Precipitation
The sample solution is warmed before the appropriate precipitant is slowly added in slight excess while the solution is constantly stirred. The precipitate-containing solution is then warmed and allowed to stand for some time. The precipitate is allowed to settle. The supernatant liquid is checked with a few drops of precipitant to ensure complete precipitation.
III) Filtration and washing of precipitate
The precipitate is poured into the funnel and washed with the appropriate wash solution. To ensure the high purity of the precipitate, the washing is checked using suitable reagents such as AgNO3 solution and BaCl2.
IV) Drying
A piece of paper is crumpled and placed over the funnel’s rim to cover the funnel containing the purified precipitate. The funnel containing the precipitate is then allowed to dry in a hot air oven for drying.
V) Ignition
When the filter paper containing the precipitate has completely dried, it is taken from the funnel and folded to completely enclose the precipitate. The packet is then placed in the weighted crucible, which is held in place by the clay pipe triangular tripod stand. The crucible is slowly heated at first. The flame is intensified to carbonize the paper once the moisture has been entirely removed. When the paper is completely carbonized, the crucible is completely heated. The crucible is covered with a lid once all of the carbon has been removed. It usually takes 50-60 minutes to complete the ignition.
VI) Heating to constant weight
Once the ignition is complete, the flame is removed, and the crucible is put in the desiccators, where it is allowed to cool for 15 to 30 minutes. The crucible and lid are then both weighed. Thereafter, the crucible substance is re-ignited for roughly 10 minutes, allowed to cool in desiccators as previously, and weighed once again. This lighting and the weighing procedure is continued until a constant weight is achieved.
Weight of precipitate = Weight of crucible content along with lid – Weight of crucible with lid
Requirements of gravimetric analysis
- The following fundamental condition must be satisfied for the gravimetric analysis based on the precipitation method to be successful.
- The component that needs to be estimated must be fully precipitated.
- The precipitate should be pure before it weighs.
- The precipitate must be suitable for handling, such as filtering, washing, weighing, etc.
- The most fundamental requirement for the gravimetric analysis is the selection of an insoluble precipitate of the constituent to be determined which has sufficient stability and is suitable for manipulation.
Classification of Gravimetric Analysis
Gravimetric analysis is broadly classified into following types:
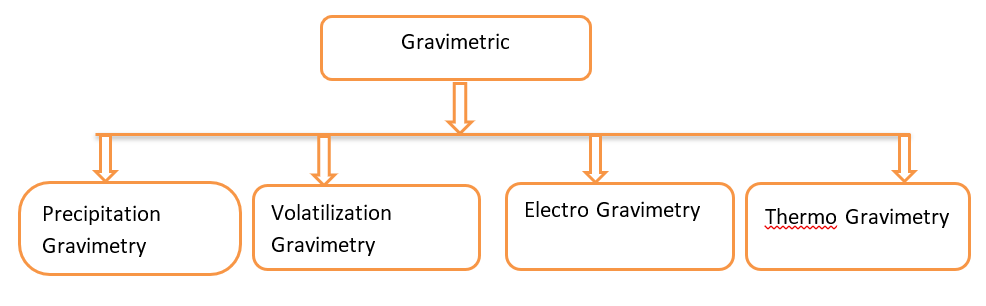
Precipitation gravimetry
Precipitation gravimetric analysis separates ions from a solution by using the precipitation process (the reaction that creates an insoluble solid product from the reaction of two soluble solid products). The chemical responsible for precipitate formation in the precipitation reaction is referred to as the precipitating agent.
For instance, the white precipitate of silver chloride is produced when aqueous silver nitrate and sodium chloride solutions react. Sodium chloride is utilized as a precipitating agent in this process.
AgNO3 + NaCl → AgCl + NaNO3
Volatilization gravimetry
The analytical technique of gravimetric volatilization separates the masses using thermal or chemical energy to determine their masses. In this method solid reactant molecules are converted into gaseous molecules using thermal or chemical energy. Different volatile gases (that can be easily evaporative), like carbon dioxide, chlorine, etc., can be separated with the help of volatilization gravimetry.
For example, the aqueous solution of sulphuric acid helps to separate carbon dioxide gas (a volatile gas) molecules from sodium bicarbonate.
2NaHCO3 (aq) +H2SO4 (aq) → 2CO2 (g) +2H2O (l) +Na2SO4 (aq)
Electro gravimetry
Electro gravimetric method is employed to separate the ions of a substance, often a metal. In this method, the analyte solution is electrolyzed. As a result of the electrolytic reduction, the analyte is deposited in the cathode.
Thermo gravimetry
Thermo Gravimetric analysis is a type of thermal analysis in which the mass of a sample is determined as a result of temperature change. It gives information on both physical and chemical phenomena, such as phase transitions, absorption, thermal degradation, and so on.
Precipitant or precipitating agent
Precipitant or precipitating agent refers to the chemical that is used to cause precipitation.A gravimetric precipitating agent should ideally react specifically or selectively with the analyte. The ideal precipitating reagent would react with the analyte to produce a precipitate in addition to specificity and selectivity.
Inorganic precipitating agent
Although they have less selectivity inorganic precipitants like S2-, PO4 3-, and CO3 2- are used for gravimetric analysis.
Organic precipitating agent
In comparison to inorganic precipitants, organic precipitants like dimethglyoxime and 8-hydroxyquinoline are more selective. They form a less soluble precipitate with the analyte. The drawback of organic precipitants is that they frequently produce an unknown-formula precipitate with the analyte, which is then burnt to produce metal oxide.
Errors in Gravimetric analysis
There are different causes of errors in gravimetric analysis these are as follows:
- Inaccurate weighing
- Incomplete and imperfect precipitation
- Ignition of precipitate at either too low or too high temperature.
- Exposure of ignited precipitate to the atmosphere.
- Use of sub standard reagent and apparatus.
- Haste and impatience
Precaution to avoid the errors in gravimetric analysis
Gravimetric analysis has several possible causes of error, however, a careful analyst with adequate technical skill would avoid frequent mistakes. For a successful gravimetric analysis, the following precaution should be used.
- Since gravimetric analysis is a lengthy and tedious process, each step must be conducted with considerable patience.
- Dilute solutions should be used for the precipitation since they prevent co-precipitation.
- The precipitant should be slowly introduced with constant stirring. This helps in large crystal growth, and the level of super-saturation is also decreased.
- Precipitation should be performed under hot conditions since precipitation is most effective in hot solutions.
- A slight excess of the precipitant should be added throughout the precipitation process, but significant excess must be avoided since they will contaminate the precipitate.
Advantages of gravimetric analysis
- Although there are numerous sources of inaccuracy in gravimetric analysis, when conducted correctly, this method remains the most accurate method for chemical analysis.
- his procedure employs low-cost equipments.
- It allows for very little instrumental error and does not necessitate a set of standards to calculate the constituent to be determined.
- It is an analytical method that helps to determine the quantity of analyte based on the density of the solid.
- This approach has been developed and standardized for practically all cations and anions.
- This technique can also be used with neutral species like iodine, carbon dioxide, water, and sulfur dioxide.
- This method has been successfully used to estimate a wider range of organic compounds, including lactose in milk products, salicylates in medications, nicotine in pesticides, and cholesterol in cereals, among others.
- Possible sources of error i.e. presence of impurities can be checked easily.
Disadvantages of gravimetric analysis
Some of the disadvantages of gravimetric analysis are as follows:
- It is a macroscopic approach since a large amount of sample is needed.
- The study of a single element or small group of elements may generally only be done via gravimetric analysis at one time.
- The study of a single element or small group of elements may generally only be done via gravimetric analysis at one time. The steps in this method are frequently complex, and a single mistake in the process can frequently cause trouble for the study.
- This method is time-consuming and difficult because it requires so many steps.
Differences between volumetric analysis and gravimetric analysis
- In contrast to volumetric analysis, which determines the amount of a desired element by measuring its volume, gravimetric analysis measures the amount of analyte by its mass.
- In volumetric analysis, the analyte’s volume is determined, whereas a gravimetric analysis determines its mass.
References
- https://byjus.com/chemistry/gravimetric-analysis/
- https://scienceready.com.au/pages/gravimetric-analysis
- https://www.pharmaguideline.com/2021/10/principle-and-steps-involved-in-gravimetric-analysis.html
- https://snscourseware.org/snscphs/files/1577033608.pdf.
- https://www.vedantu.com/chemistry/gravimetric-analysis
