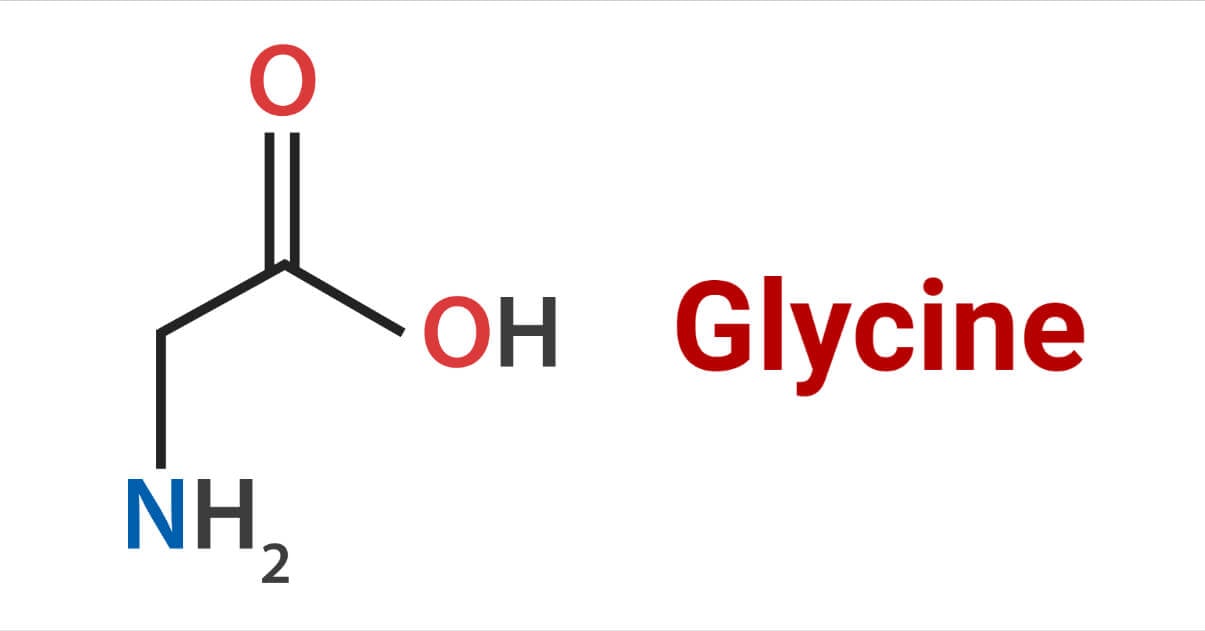
Glycine is a sweet-tasting amino acid obtained from the hydrolysis of protein. It is a nonessential amino acid present in mammals. Mammals can synthesize glycine from the amino acids serine and threonine, and it is also present in certain animal excretion like it is present in horse urine as hippuric acid C6H5CONHCH2COOH (Benzoyl glycine).
Among proteinogenic amino acids, glycine is an achiral amino acid. As it contains two hydrogen atoms on both sides, it can fit into a hydrophilic and hydrophobic environment.
Preparation of Glycine
- Reaction of Chloroacetic acid with ammonia: Chloroacetic acid reacts with ammonia to produce glycine by replacing the chlorine atom in the acid with -NH2.
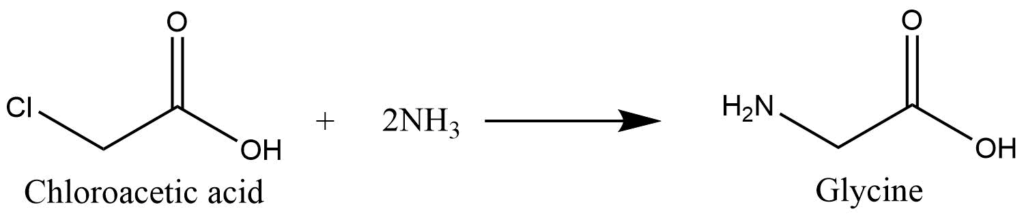
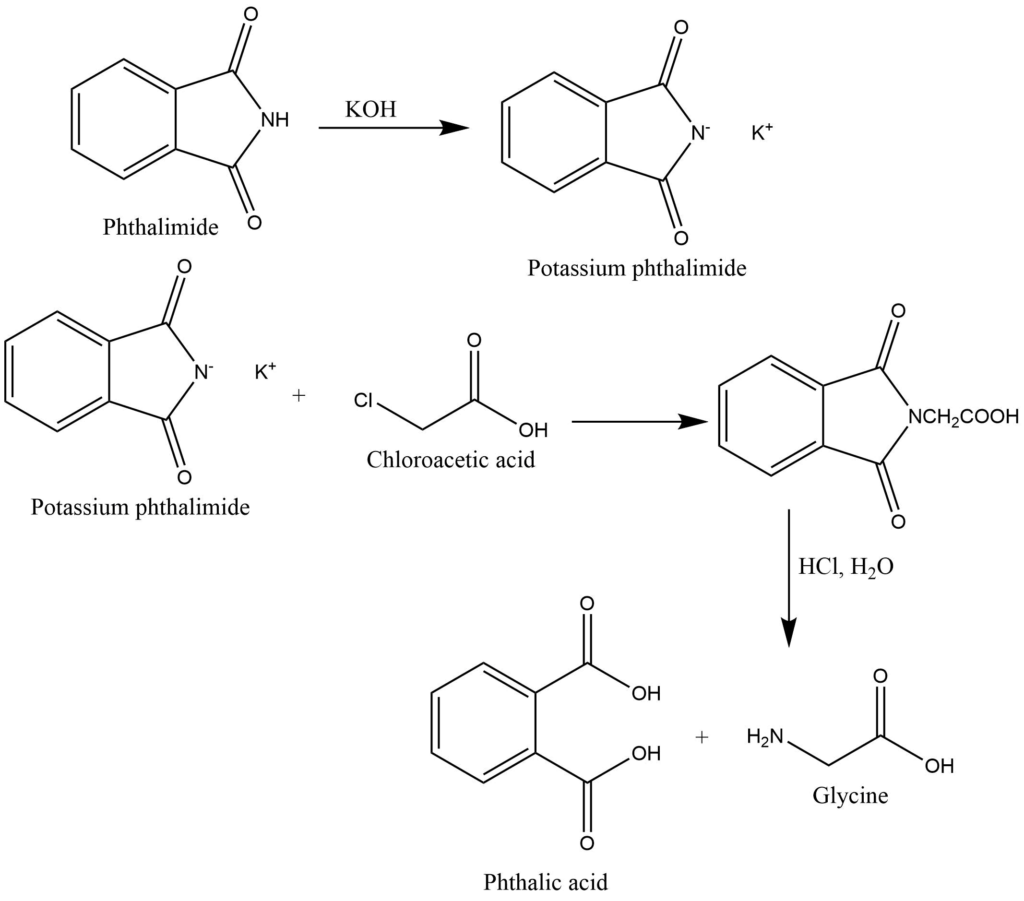
2. By Gabriel’s Phthalimide synthesis: Phthalimide reacts with alcoholic KOH to give potassium phthalimide. Potassium phthalimide on heating with chloroacetic acid and further hydrolysis produces glycine.
Physical Properties of Glycine
- It is a white crystalline solid having a sweet taste.
- It is soluble in water but insoluble in ethanol and ether.
- Due to its acidic nature, this acts as a buffer solution at a pH of 6.00.
- It melts with decomposition at 289-292oC.
Chemical Properties of Glycine
A. Reaction due to carboxylic acid:
1. Reduction: Glycine reduces to the alcohol by an aqueous solution of lithium aluminum hydride.
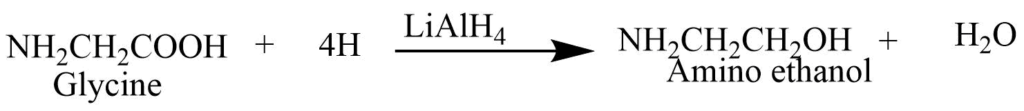
2. Decarboxylation reaction: On heating with soda lime or barium hydroxide, glycine produces the methyl amine.
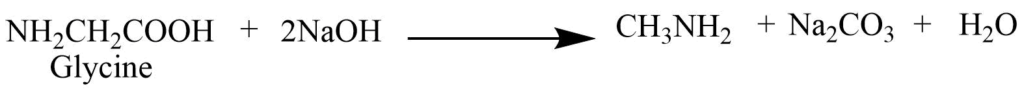
3. Reaction with alcohols: It reacts with alcohol in presence of inorganic acid to form an ester.
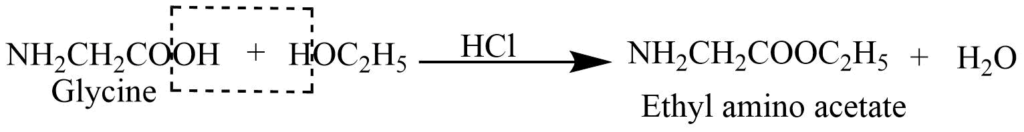
B. Reaction due to amino group
1. Salt formation with acid: On reaction with acids like hydrochloride glycine forms salt such as glycine hydrochloride. Such type of salt dissolves in water to give a solution whose pH values are higher than that of glycine solution. This is because the basicity of the amino group has been neutralized.

2. Acetylation: Glycine reacts with acetyl chloride or acetic anhydride to give acetyl derivative and with benzyl chloride to form benzoyl chloride or hippuric acid.
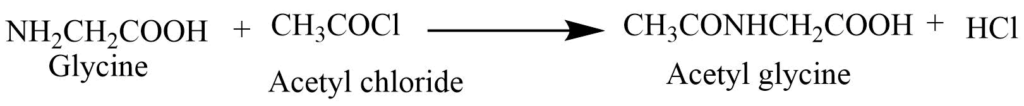
3. With nitrous acid: The reaction of glycine with nitrous acid replaces the amino acid present on glycine with a hydroxyl group with nitrogen evolution.
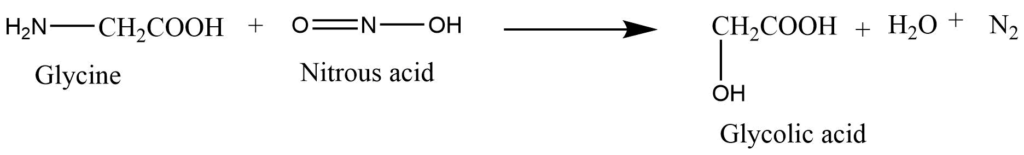
4. With nitrosyl chloride: Glycine reacts with nitrosyl chloride to give chloroacetic acid.

C. Reaction due to both amino and carboxyl groups
1. Formation of dipolar ion or inner salt or Zwitter ion: In an aqueous solution glycine exists as a dipolar ion. Such an ion is known as ampholyte or Zwitter ion. The dipolar ion accounts for the absence of acidic or basic properties of amino acids. The high melting point and insolubility in organic solvent also indicate the existence of inner salt in the solid state.

2. Isoelectric point: The Zwitter ion may either lose or gain H+ ion thus acting as acid or base.
In an aqueous solution, the Zwitter ion tends to lose a proton, so on electrolysis, the negative glycine ion migrates to the anode.
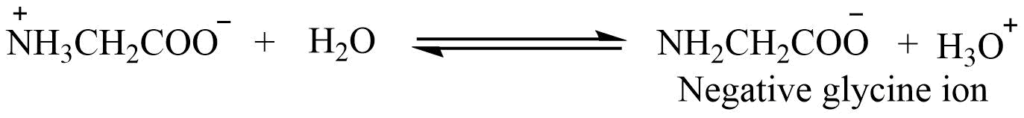
When mineral acid is added, Zwitter is converted to positive ions and migrated towards cathode during electrolysis.

3. Effect of heat: Glycine on heating forms diketo piperazine, which is a cyclic diamide.
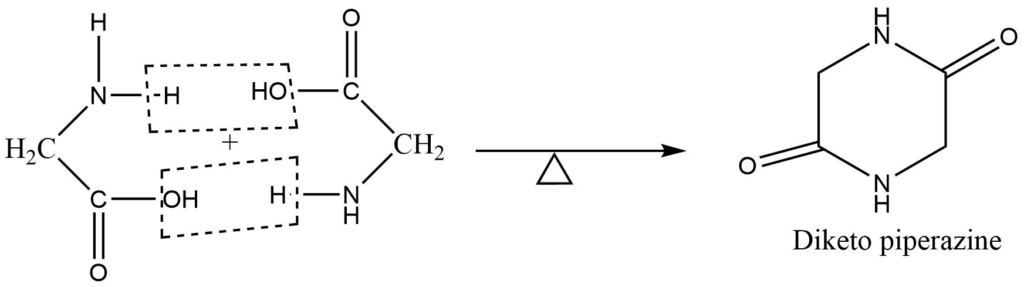
Uses of Glycine
- It is used as a food additive.
- It is used in photography.
- It is used for the manufacture of resins and varnishes.
- It is used in medicine for muscular disorders and also for the treatment of stroke and schizophrenia.
- In industry, it involves in metal complexing and finishing
- It is used as a buffering agent in cosmetics and analgesics.
References
- https://byjus.com/chemistry/glycine-structure/
- https://www.worldofchemicals.com/chemicals/chemical-properties/glycine.html
- https://www.aakash.ac.in/important-concepts/chemistry/glycine-structure
- https://collegedunia.com/exams/glycine-structure-uses-preparation-solved-examples-chemistry-articleid-4617
- https://www.newworldencyclopedia.org/entry/Glycine
