Interesting Science Videos
Glucose Definition
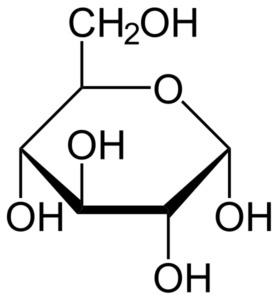
Figure: Glucose Structure.
Glucose is a simple form of sugar that is the primary source of energy in the living systems.
- Glucose is the most abundant monosaccharide on earth, which occurs and is utilized by different organisms throughout the world.
- The primary sources of glucose on earth are green plants and algae through the process of photosynthesis by utilizing carbon dioxide and water.
- The monomeric glucose is utilized as immediate energy by living beings, but it can also be stored as a polymer for future use.
- Glucose is composed of six carbon atoms and an aldehyde functional group, thus it is an aldohexose, a category within monosaccharides.
- The term glucose is derived from the French term ‘glukos’, meaning sweet, indicating the sweet taste of the molecule.
- Glucose molecules can exist either in an open-chain or acyclic structure or a ring or cyclic structure.
- Glucose exists in two enantiomeric forms; D-glucose and L-glucose, where the D-glucose, also called dextrose, occurs widely in nature, but the L-form is rare.
- Since glucose is a simple sugar or monosaccharide, it can be obtained by the hydrolysis of different polymeric sugars like lactose and sucrose.
- The molecular formula of glucose is C6H12O6 or H-(C=O)-(CHOH)5-H, where the five hydroxyl groups are arranged in a specific way along the six-carbon back.
- Glucose is the most widely used aldohexose in most living organisms, which can be explained by the lower tendency of the molecule to react nonspecifically with the amine group of proteins.
- The lower reactivity towards proteins is attributed to the fact that glucose can exist in the more stable cyclic form when compared to other aldohexoses.
- In living systems, glucose usually doesn’t occur in its free form but as polymers in order to reserve energy.
Sucrose Definition
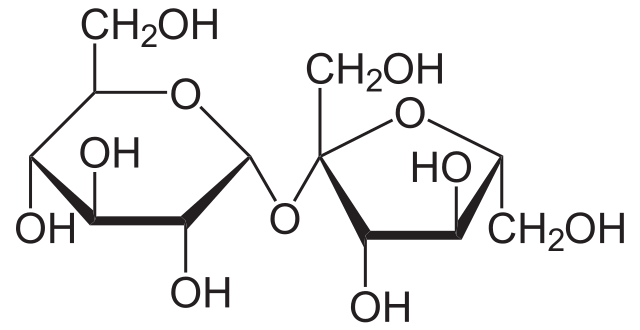
Figure: Sucrose Structure.
Sucrose is a disaccharide composed of fructose and glucose in a 1:1 ratio as its two constituent monosaccharides.
- Sucrose is obtained naturally from plants like sugarcane and maple in a large amount, and most of it is entirely used as food.
- Sucrose is chemically more complex than glucose as it can be broken down into smaller, simpler units.
- The term sucrose is derived from the French term ‘sucre’, meaning sugar, indicating the source and nature of the compound.
- The monomeric units in sucrose are linked to each other via an ether bond between the C1 on the glucose and C2 on the fructose.
- The glycosidic bond in sucrose is formed between the reducing ends of the monomers, which is quite unusual from other disaccharide linkages.
- The glycosidic bond in sucrose inhibits the bonding of the monomers to other monomers and prevents the reaction of sucrose with cellular macromolecules.
- The breakdown of sucrose to release the monomeric units is termed hydrolysis, but the hydrolysis of sucrose is very solution as a sucrose solution can stay without hydrolysis for years.
- The hydrolysis can, however, be accelerated by adding enzymes called sucrase. Hydrolysis of sucrose can also be brought about by the addition of acid as it degrades the acetal bond between the monomers.
- Sucrose is the end product of photosynthesis and, thus, is found naturally in different plants along with fructose.
- In other living beings, sucrose doesn’t occur as a storage sugar but occurs as an intermediate in different biochemical pathways.
- Sucrose is a non-reducing sugar as the reducing ends of the monomers present in sucrose are involved in the formation of the glycosidic bond.
13 Key Differences (Glucose vs Sucrose)
| Characteristics | Glucose | Sucrose |
| Definition | Glucose is a simple form of sugar that is the primary source of energy in the living systems. | Sucrose is a disaccharide composed of fructose and glucose in a 1:1 ratio as its two constituent monosaccharides. |
| Complexity | Glucose is a simple sugar. | Sucrose is more complex than glucose. |
| Type | Glucose is a monosaccharide. | Sucrose is a disaccharide. |
| Chemical Formula | The chemical formula of glucose is C6H12O6. | The chemical formula of sucrose is C12H22O12. |
| Reducing nature | Glucose is a reducing sugar as it has a free reducing group. | Sucrose is a non-reducing sugar as it has no free reducing group. |
| Molecular weight | Glucose has a smaller molecular weight. | Sucrose has a higher molecular weight. |
| Composed of | Glucose is monomeric sugar. | Sucrose is composed of glucose and fructose. |
| Glycosidic bond | The glucose molecule doesn’t have a glycosidic linkage. | Sucrose molecule consists of a single glycosidic linkage. |
| Reactivity | Glucose is more reactive than sucrose. | Sucrose is less reactive than glucose. |
| Occurrence | Glucose is the most abundant sugar. | Sucrose is not as abundant as glucose. |
| Structure | Glucose exists in a simpler and unbranched structure. | Sucrose might exist in a complex and branched structure. |
| Glucose has a single ring structure. | Sucrose has two ring structures. | |
| Solubility | Glucose is soluble in water. | Sucrose is insoluble in water. |
References and Sources
- Jain JL, Jain S and Jain N (2005). Fundamentals of Biochemistry. S. Chand and Company.
- 2% – https://wiki2.org/en/Sucrose
- 2% – https://quizlet.com/244811195/carbs-tbich-flash-cards/
- 2% – https://en.wikipedia.org/wiki/Glucose
- 2% – https://en.wikipedia.org/wiki/D-Glucopyranose
- 1% – https://www.reference.com/science/takes-place-during-hydrolysis-sucrose-a0bba9c79f1ce9cf
- 1% – https://www.ausetute.com.au/redsugar.html
- 1% – https://www.answerout.com/sucrose-is-a-disaccharide-composed-of-the-monosaccharides-glucose-and-fructose-the-hydrolysis-of-sucrose-by-the-enzyme-sucrase-results-in-2/
- 1% – https://en.wikipedia.org/wiki/Sucrose
- 1% – https://en.wikipedia.org/wiki/Photosynthetic
- 1% – https://en.m.wikipedia.org/wiki/Glucose
- 1% – https://diabetestalk.net/blood-sugar/difference-between-glucose-and-fructose-structure
- 1% – https://diabetestalk.net/blood-sugar/difference-between-glucose-and-sucrose-chemically
- <1% – https://www.reference.com/science/end-product-photosynthesis-a76e7b5e92a3d21b
- <1% – https://www.answers.com/Q/Glycosidic_linkage_between_glucose_and_fructose
- <1% – https://pediaa.com/what-is-the-difference-between-blood-sugar-and-glucose/
- <1% – https://en.wikipedia.org/wiki/Sugar
- <1% – https://diabetestalk.net/blood-sugar/what-is-the-difference-between-the-structure-of-glucose-and-fructose
- <1% – https://diabetestalk.net/blood-sugar/is-glucose-a-polymer
- <1% – https://chestofbooks.com/food/science/Experimental-Cookery/Effect-Of-Acid-Upon-Sugars-And-Hydrolysis-Of-Sugars.html
- <1% – https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Book%3A_The_Basics_of_GOB_Chemistry_(Ball_et_al.)/16%3A_Carbohydrates/16.06%3A_Disaccharides
- <1% – https://biology.stackexchange.com/questions/24668/why-should-plants-transform-glucose-into-sucrose-before-transporting-it-to-other
