Gas chromatography (GC) is a separation technique in which volatile, thermally stable solutes migrate through a column containing a stationary phase at rates determined by their distribution ratios. In a wide range of mixtures, from the simplest (such as purity tests of individual compounds) to the most complex (such as petrochemical assays of samples consisting of hundreds of individual components), GC provides separation and quantitative analysis for volatile, thermally stable compounds.
The term “gas chromatography” can also refer to vapor-phase chromatography (VPC) or gas-liquid partition chromatography (GLPC).
Interesting Science Videos
What is Gas Chromatography?
Gas chromatography (GC) is one of the most versatile and widely used laboratory analytical techniques. In gas chromatography, a sample’s constituent parts are dissolved in a solvent and turned into vapor in order to separate the analytes by dividing the sample between two phases: a stationary phase and a mobile phase.
A chemically inert gas known as the mobile phase is used to transport analyte molecules through the heated column. One of the only types of chromatography that does not use the mobile phase to interact with the analyte is gas chromatography.
GC relies on the varying affinities of vapor components for surfaces. A mixture is first vaporized and then picked up by an inert gas in a gas chromatograph. A tube or “column” that was initially filled with tiny, solid particles is then pushed with this carrier gas. Some compounds interact with the solid surfaces more strongly than others due to their unique chemical properties, slowing their progress through the column. A specialized detector is located at the column’s end, and as compounds leave the column it emits a signal whose intensity roughly reflects the relative concentration of each component.
Each component of the mix has a peak when the signal is plotted on graph paper (or, in later years, on a computer screen). For any given sample, provided it is run through the column in the same manner, the pattern of peaks, or “chromatogram,” is repeatable.
History
Anthony T. James and Archer J. P. Martin of the National Institute for Medical Research in London introduced GC in 1951–1952. The method was developed from earlier chromatography research conducted by several scientists, including that for which Martin and Richard L. M. Synge were awarded the 1952 Nobel Prize in Chemistry.
Principle of Gas Chromatography
The separation of volatile components of a sample between the mobile gaseous phase and stationary liquid phase is the principle behind gas chromatography. A substance’s solubility between the stationary liquid phase and the gaseous mobile phase depends on its partition coefficient.
The parts of the sample that are divided into the gas phase emerge first, followed by the rest of the sample. As a result, the sample’s components are separated using the partition chromatography principle. A liquid layer covering the stationary phase is known as the stationary phase. While the gas in the mobile phase is stable and inert.
Types/ Modes of Gas Chromatography
Gas chromatography has two different operating modes:
- Gas-liquid chromatography (GLC), which uses a liquid stationary phase that solutes can dissolve in, with partition acting as the sorption process. The order of elution from that of increasing boiling points may change depending on specific interactions of solutes with the stationary phase. The fact that there are so many different alternative stationary phases makes it possible to analyze a wide variety of sample types, making GLC by far the more popular GC mode.
- Gas-solid chromatography (GSC) uses a solid, occasionally polymeric, sorbent as the stationary phase; surface adsorption is the sorption process. GSC has a small number of specialized uses; it is typically used to analyze mixtures of gases or solvents with low relative molecular masses.
Instrumentation
Instrumentation for GC has continued to advance over the past 15 years, albeit slowly. An oven’s exquisite design combined with a clever electronic flow controller of the carrier gases results in a retention time RSD that is better than 0.05%. Peak areas can be measured with a comparable level of accuracy, especially when internal standards are used. All functions and parameters are now routinely controlled by computers. The biggest development in the pneumatic field has been the electronic flow controller. Agilent and Perkin Elmer have created algorithms to automatically tune the instrument for the best performance.
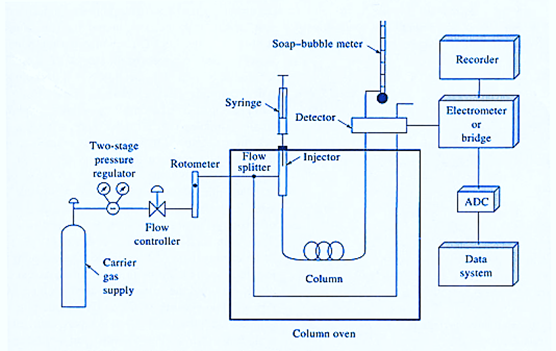
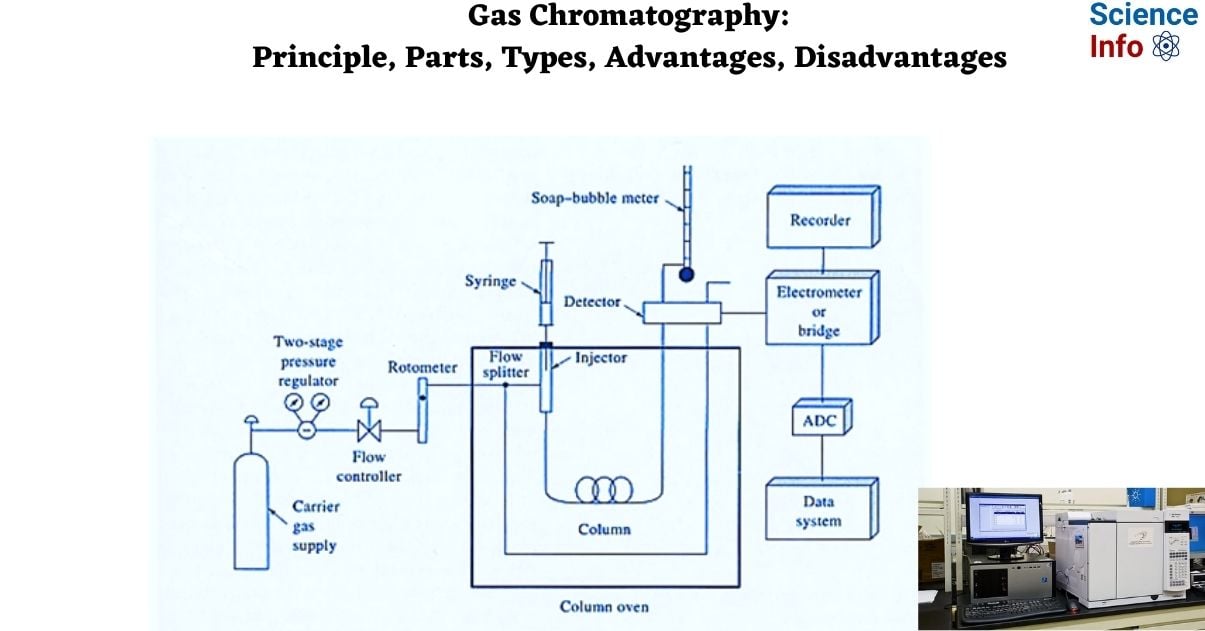
The following essential components are found in a good gas chromatography machine:
- Carrier Gas Supplier
- Pressure regulator
- Sample injection port
- Gas chromatography column
- The separation column
- Stationary phase
- Detector
- Signal recorder
Carrier Gas Supplier
It is a mobile phase consisting of inert gases such as Hydrogen, Helium, Argon etc. The choice of carrier gas depends on nature of the sample, consumption, column efficiency, type of detector and so on.
Mobile phase
The only function of the mobile phase—also referred to as the carrier gas—is to move solutes through the column; it has no effect on chromatographic selectivity. It should preferably be inexpensive and inert, non-toxic, and non-flammable.
Both nitrogen and helium are routinely used, with packed columns for nitrogen and capillary (open tubular) columns for the former (vide infra). Due to faster mass transfer, helium provides better chromatographic efficiency (reduced band broadening)
In order to prevent undesirable chemical changes to sample components and stationary phases, as well as negative effects on detector performance, the carrier gas must be purified by passing it through appropriate adsorbents.
Pressure regulator
The range for pressure adjustment is 1 to 4 atmospheres, and the range for flow control is 1 to 1000 liters per minute of gas. A needle valve mounted on the base adjusts flow valves. Due to their high thermal conductivity, helium, argon, nitrogen, and hydrogen may be the preferred carrier gas.
Sample injection port
A self-sealing silicon rubber septum in a heated metal receives samples via a microsyringe injection. The metal bock is an electrical heater that is heated. For the injection of a sample, we used injection ports of various sizes.
Gas chromatography column
By coiling tubing into an open spiral, a gas chromatography column can be created. In order to operate at high temperatures, we used copper or stainless steel. The inner diameter of the chromatographic column affects the velocity of the carrier gas flow rate. The column typically measures 2 meters in height.
The separation column
The metal column, which is bent into a U-shape or wound into an open spiral, is the essential component of gas chromatography. Here, the sample mixture is divided, and it is then brought to the detector. Depending on the need, different-sized columns are used. For instance, packed columns, capillary columns, and open tubular columns are frequently used. In GC, packed columns and capillary columns are the two types of columns that are utilized.
Detector
The detector can produce an electrical signal by spotting the entry of components from the column. The two main categories of detectors used in gas chromatography are pressure and temperature detectors.
In gas chromatography equipment, the detector is placed close to the column to prevent liquid condensation or to find the sample before it decomposes. We primarily used a thermal conductivity detector (TCD) or a flame ionization detector in a packed column gas chromatography setup (FID).
Of these, TCD is the most well-liked. It is best to use a flame ionization detector (FID) when a stream splitter has effectively attenuated the effluent. Four heat-sensing components, either resistance wires or thermistors, are found in TCD detectors. The electrical resistance of the thermometers, which are electronic semiconductors made of fused metal oxides, changes with temperature.
Recorder
The purpose of the recorder is to capture and amplify signals from detectors to produce an electronic response in the shape of a graph known as a chromatogram. Typically, the recorder should have a fast response pen and be 10 mv (full scale) (1 sec or less). To attenuate the strong signals, the recorder should be connected with a number of high-quality resistances placed across the input.
Advantages of Gas Chromatography
The main advantage of gas chromatography is that it allows you to identify unknown substances in a volatile compound. This allows us to gain a better understanding of the compound and its properties. Gas chromatography is a very flexible scientific method that can be used to measure hundreds of different compounds. Additionally, it is a very reliable method that can be used in conjunction with others, such as mass spectrometry. This broadens its applications and the range of its uses.
Some important advantages are listed below:
- Gas chromatography’s main advantage is its high sensitivity, resolution, and separation ability, which allows it to separate a wide range of volatile compounds.
- In comparison to other chromatographic methods, gas chromatography can analyze a sample a lot faster.
- It can detect extremely low concentrations using a very small amount of sample injection and highly sensitive detectors.
- It is a reliable separation method with a high signal-to-noise
- There are various types of GC columns that come in a wide range of diameters and lengths depending on the needs of the molecule.
- Gas chromatography offers relatively high precision, accuracy, and reproducible results because it is simple, automated, and has quick data analysis.
- Even during chromatographic runs, operational parameters like flow rate, temperature, and pressure, etc., are simple to modify.
- This technique allows for the separation, determination, and identification of numerous compounds with tiny differences in their boiling points.
- It is possible to conduct both qualitative and quantitative analysis concurrently.
- When connected to a mass spectrometer, both its resolution and sensitivity are significantly increased. As a result, even if they are present in trace amounts, we can separate and distinguish between very similar species using gas chromatography.
- Additionally, because the data is digital, there is less chance that it will disappear or deteriorate.
Disadvantages of Gas Chromatography
To avoid any scientific bias, we must also consider the drawbacks of gas chromatography. A few of these are listed below:
- This method is not appropriate for the analysis of non-volatile species or species that are not thermally stable.
- In order to prevent the temperature from dropping too low during gas chromatography, the environment must be carefully monitored.
- Care must be taken when handling and storing the capillary tubes
- With the exception of the MS, all GC detectors are destructive.
- Selectivity in HPLC or TLC is also preferable due to the simplicity of changing the mobile phase. In GC, you can only change the column and oven temperatures; the mobile phase cannot be changed because it is constantly being injected with carrier gas (helium, nitrogen).
- Care must be taken when using hydrogen gas, which is used to create flames because it is highly flammable.
- Individual sample components cannot be recovered.
Applications of Gas Chromatography
Here are few applications for gas chromatography:
- Gas chromatography is used in pharmaceuticals to ensure that the correct ingredients are used for a drug and that there are no contaminants in the batch.
- If an unknown drug is discovered, gas chromatography can be used to identify its chemical constituents, allowing us to better understand its effects.
- Gas chromatography can be used to analyze air samples and determine what pollutants are present.
- GC can be used in forensics to examine the evidence found at a crime scene. For instance, in an arson case, the chemical composition of fire residues can be examined to determine how the fire was started.
- GC can be used to identify the contaminants in food if there is a suspicion of contamination.
References
- https://www.acs.org/education/whatischemistry/landmarks/gas-chromatography-mass-spectrometry
- https://www.technologynetworks.com/analysis/articles/gas-chromatography-how-a-gas-chromatography-machine-works-how-to-read-a-chromatograph
- https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/gas-chromatography
- https://microbenotes.com/gas-chromatography/

Dear Jyoti Bashyal which types of Gas chromatography supports O2 analyzing? could you please advise.
In the context of gas chromatography, a sample undergoes vaporization and subsequent separation into its constituent parts within a chromatographic column. The detection of these separated components is carried out by a detector. Certain gas chromatography detectors may exhibit reduced sensitivity to oxygen compared to other compounds. Notably, commonly employed flame ionization detectors (FID) may not offer optimal sensitivity for oxygen. Consequently, specialized detectors like thermal conductivity detectors (TCD) and electrolytic conductivity detectors (ELCD) are frequently utilized in gas chromatography for precise oxygen analysis. Additionally, dual-column gas chromatography is applied for analyzing oxygen and argon, with factors such as column choice, stationary phase selection, and carrier gas composition requiring careful consideration for specific analyses.