A fuel cell is a type of galvanic cell that uses traditional combustible fuels, most commonly hydrogen or methane, which are continuously fed into the cell along with an oxidant. (A flow battery is another, a less well-known name for a fuel cell.) Fuel and oxidant undergo the same redox chemistry as when they are combusted within the cell, but through a catalyzed electrochemical that is significantly more efficient.
Interesting Science Videos
What is Fuel Cell?
A fuel cell is a galvanic cell or electrochemical power source, which converts the energy of chemical reactions into electrical energy. It is an electrochemical cell in which one electrode gives up electrons while the other electrode gains electrons.
Fuel cells are increasingly being used to power buses and cars instead of gasoline. The fuel is stored in the vehicle’s tanks, and the oxygen comes from the air. The energy released by a fuel cell generates a voltage that can be used to power the vehicle’s electric motor.
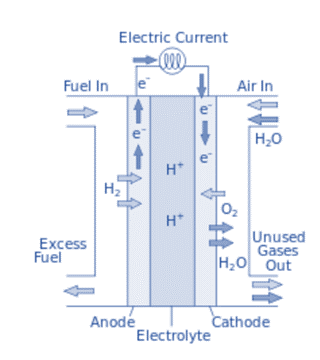
Working of Fuel Cell
A fuel cell can use the reaction between hydrogen and oxygen to generate electricity. This type of cell was used in the Apollo space program for two purposes: it served as a fuel source as well as a source of drinking water (the water vapor produced from the cell, when condensed, was fit for human consumption).
This fuel cell worked by passing hydrogen and oxygen through carbon electrodes into a concentrated solution of sodium hydroxide. The cell reaction is expressed as follows:
Cathode Reaction: O2 + 2H2O + 4e– → 4OH–
Anode Reaction: 2H2 + 4OH– → 4H2O + 4e–
Net Cell Reaction: 2H2 + O2 → 2H2O
However, the reaction rate of this electrochemical reaction is quite slow. This problem is solved by using a catalyst, such as platinum or palladium. To increase the effective surface area, the catalyst is finely divided before being incorporated into the electrodes.
Fuel cell setup
A fuel cell performs the fundamental function of generating electricity, which can also be used to power anything from a light bulb to an entire city. A simple chemical reaction within a fuel cell is responsible for the generation of electricity, which would eventually return to the cell to complete the electric circuit.
The anode initiates this chemical reaction by introducing hydrogen atoms. At this point, a chemical reaction depletes the hydrogen atoms of their electrons. The hydrogen atoms now have a positive electric charge. The remaining negatively charged electrons conduct current through the wires. At the cathode, oxygen atoms are introduced. As a result they react with the electrons left over from the hydrogen atoms.
Depending on the type of cell, the oxygen atoms and negatively charged electrons would either combine with the positively charged hydrogen ions at this point or after passing through the anode.
Types of Fuel Cells
Fuel cells of various types have been developed. They are generally classified based on the electrolyte used, because the electrolyte determines a system’s operating temperature and, in part, the type of fuel that can be used.
Hydrogen – Oxygen Fuel Cell
The hydrogen-oxygen fuel cell is one type of fuel cell. The half-reactions take place when hydrogen gas and oxygen gas are bubbled through two porous platinum-coated electrodes. Electrons flow from the negative pole to the positive pole via the external circuit. Their energy is used to power an electric motor or another device as they do so. A typical hydrogen fuel cell, for example, employs graphite electrodes embedded with platinum-based catalysts to accelerate the two half-cell reactions.
| H2 + 1/2 O2 → H2 | ΔGf = 229 kJ/mol |
| H2 → 2 H+ + 2 e– | E0= 0.00 V |
| O2 + 4 e– + 4 H+ → 2 H2O | E0= 1.2V |
The source of hydrogen is a major issue with hydrogen fuel cells. Because the amount of H2 in the atmosphere is so low, it must be produced.
The majority of hydrogen is currently produced from natural gas and petroleum. It can also be made from coal through the water gas and water gas shift reactions. All of these processes use the energy stored in fossil fuels and thus emit CO2.
Electrolysis can produce hydrogen from electricity generated by other sources (such as nuclear power, hydropower, or solar energy). However, this is the opposite reaction of the hydrogen fuel cell reaction. H2 is clearly a medium for energy transfer rather than the fuel.
Alkaline fuel cells
These are devices in which the electrolyte is an aqueous solution of sodium hydroxide or potassium hydroxide. Almost always, the fuel is hydrogen gas, with oxygen (or oxygen in the air) acting as the oxidizer. However, if the by-product oxides were efficiently removed and the metal was fed continuously as a strip or powder, zinc or aluminum could be used as an anode.
- This was the fuel cell that served as the primary source of power for the Apollo space program.
- An aqueous alkaline solution is used in these cells to saturate a porous matrix, which is then used to separate the electrodes.
- Operating temperatures are quite low (approximately 90°C).
- These cells are extremely effective. Along with electricity, they generate heat and water.
Phosphoric acid fuel cells
The electrolyte in these cells is orthophosphoric acid. Phosphoric acid which is used as an electrolyte in these fuel cells channels the H+. On a small scale, phosphoric acid fuel cells have been proposed and tested for local municipal power stations as well as in remote-site generators.
- The electrodes are made of catalyzed carbon and are arranged in back-to-back pairs to form a series generation circuit.
- These cells operate at temperatures ranging from 150°C to 200°C.
- Because phosphoric acid is non-conductive, electrons must travel to the cathode via an external circuit.
- Because the electrolyte is acidic, the components of these cells corrode or oxidize over time.
Molten carbonate fuel cells
The fuel is made up of a mixture of hydrogen and carbon monoxide produced by water and fo ssil fuel. Molten potassium lithium carbonate serves as the electrolyte. Molten carbonate fuel cells should be useful in both small and large power plants.
- An operating temperature of approximately 650 °C (1,200 °F) is required.
- Because of the high operating temperature and the presence of the carbonate electrolyte, the anode, and cathode of this cell are prone to corrosion.
- These cells can also run on carbon-based fuels like natural gas and biogas.
Solid oxide fuel cells
Solid oxide fuel cells are similar to molten carbonate devices in some ways. However, the majority of the cell materials are special ceramics containing nickel. The electrolyte is a yttria-treated ion-conducting oxide such as zirconia. As with molten carbonate cells, the fuel for these experimental cells is thus expected to be hydrogen combined with carbon monoxide. While the path of the internal reactions would be different, the cell products would be water vapor and carbon dioxide.
- Solid oxide fuel cells would be designed for use in central power plants where temperature variation could be efficiently controlled and fossil fuels were available.
- These fuel cells are extremely efficient and relatively inexpensive (theoretical efficiency can even approach 85%).
- These cells’ operating temperatures are extremely high (lower limit of 600°C, standard operating temperatures lie between 800°C and 1000°C).
- Because of their high operating temperatures, solid oxide fuel cells are only suitable for stationary applications.
| H2 + ½O2 → H2O | (overall reaction) |
| H2 + O2- → H2O + 2e– | (oxidation reaction) |
| ½O2 + 2e– → O2- | (reduction reaction) |
Methanol Fuel Cell
Methanol is a liquid fuel that can be produced through fermentation from renewable resources. The electrochemical reactions described below are used in direct methanol fuel cells:
| Anode reaction | CH3OH + H2O → CO2 + 6 H+ + 6 e– |
| Cathode reaction | 3/2 O2 + 6 H+ + 6 e– → 3 H2O |
| Cell reaction | CH3OH + 3/2 O2 → CO2 + 2 H2O |
- Carbon dioxide, a greenhouse gas, is produced during the electrochemical reaction. The anodic reaction necessitates the use of an expensive, precious metal catalyst. A typical alloy is a ruthenium and palladium. However, another disadvantage of this fuel cell is methanol’s toxicity.
- Methanol is also compatible with hydrogen fuel cells. Steam reforming methanol at 250 degrees Celsius produces CO2, H2, and a trace of CO.
The Advantages of Fuel Cells
More efficient: Because fuel cells convert chemical energy directly into electrical energy, they are far more efficient than traditional combustion engines. Fuel cells outperform other powering mechanism devices in terms of effectiveness. They have a direct channel for energy conversion, rather than a double conversion process. As a result, these are widely recommended.
Reduces wasteful emissions: Unlike other cells that emit greenhouse gases during the energy conversion process, fuel cells are a significant improvement because the only emissions they emit are heat and water. As a result, it is beneficial to the environment.
More stable: The fuel cells ensure that various parts within and around the cell move as little as possible. As a result, they are more dependable and convenient than traditional cell phones.
Protects natural resources: In fuel cells, the process of atom separation and energy generation is very clean and ergonomic. As a result, it is beneficial to natural resources.
Complementary: Fuel cells are by far the most ergonomic solution when combining the cell with other technologies. This combination of turbines and solar panels is also possible. As a result, complementary in nature.
Scalable: The fuel cells can generate electricity ranging from a few milliwatts to many megawatts. It also aids in the powering of various devices such as cell phones and homes. As a result, they are scalable.
References
- Handbook of Fuel Cells: Fundamentals, Technology, Applications (four volumes), W. Vielstich, A. Lamm, and H. Gasteiger (editors), Wiley, Chichester, UK, 2003.
- https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Chemistry_for_Changing_Times_(Hill_and_McCreary)/08%3A_Oxidation_and_Reduction/8.03%3A_Electrochemistry-_Cells_and_Batteries
- https://www.britannica.com/technology/fuel-cell/Types-of-fuel-cells
- Fuel Cells: Problems and Solutions, V. S. Bagotsky, Wiley, Hoboken, NJ, 2009.
- https://knowledge.electrochem.org/encycl/art-f03-fuel-cells.htm
- https://byjus.com/chemistry/fuel-cell/
- https://www.toppr.com/guides/chemistry/electrochemistry/fuel-cells/
- Fundamentals of Electrochemistry with Application to Direct Alcohol Fuel Cell Modeling, DOI: 10.5772/intechopen.71635
