Fractional distillation is a method of separating the various components of a chemical mixture into different parts (called fractions) based on their boiling points. In fractional distillation, a liquid mixture is evaporated to produce a mixture of constituents, from which the desired one is purified. As some of the vapor is condensed and returned as a liquid, this technique is sometimes called rectification. In this process, fractionating columns are used for the separation of miscible volatile liquids with similar boiling points. In this process, chemical compounds are separated by heating them to a temperature where one or more portions of the mixture evaporate. Fractional distillation is widely used to purify substances and separate mixtures to acquire their components.
In oil refineries, for example, fractional distillation is used to separate crude oil into usable molecules containing distinct types of hydrocarbons with different boiling points. More carbon atoms are found in crude oil compounds or fractions with higher boiling points.
The working mechanism of Fractional Distillation
Fractional distillation is used to separate the mixture of the components of the liquid into pure form in the following ways:
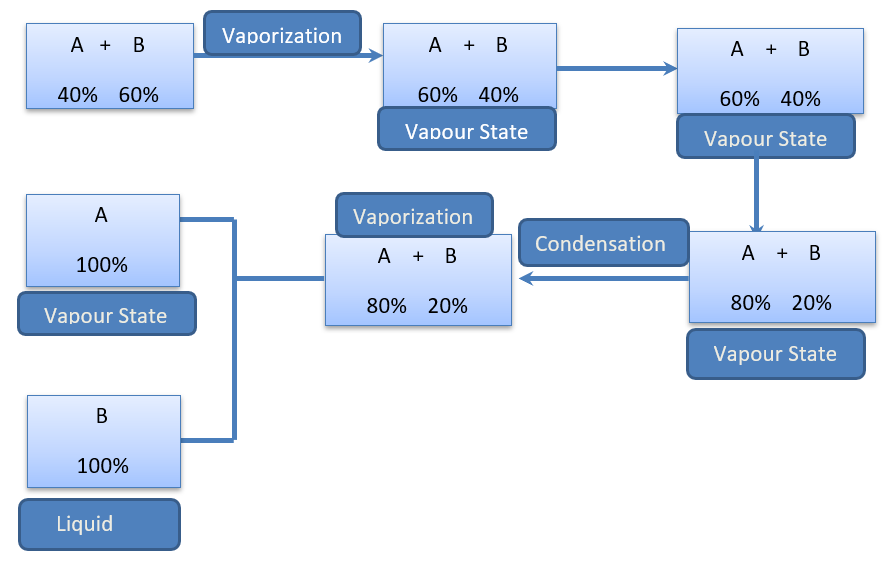
a. Normally, the vapor of any liquid mixture does not retain the same composition as the liquid. When the specified mixture is heated, the lower boiling point liquid boils and transforms into vapor.
b. The more volatile components are more likely to persist in the vapor state than in the liquid state. The mixture passes through several distillations and condensations, resulting in distinct fractions.
c. The more volatile substances increase their vapor state after heating. However, as the vapor is liquefied, it becomes liquid.
d. Distillation refers to the combined processes of initial vaporization and subsequent condensation. When this procedure is continued, a more volatile chemical will eventually remain in the liquid state in its pure form.
In this way, the various components of the liquid-liquid mixture can be separated in a pure form by using the fractional distillation method.
Principle of Fractional Distillation
The fundamental concept of fractional distillation is that different liquids boil and evaporate at different temperatures. When the liquid mixture is heated, the substance with the lower boiling point starts boiling first and converts into vapor.
Let us consider a liquid-liquid mixture of A and B where liquid A is more volatile than liquid B. This mixture can be separated by the following process:
Laboratory Procedure of Fractional Distillation
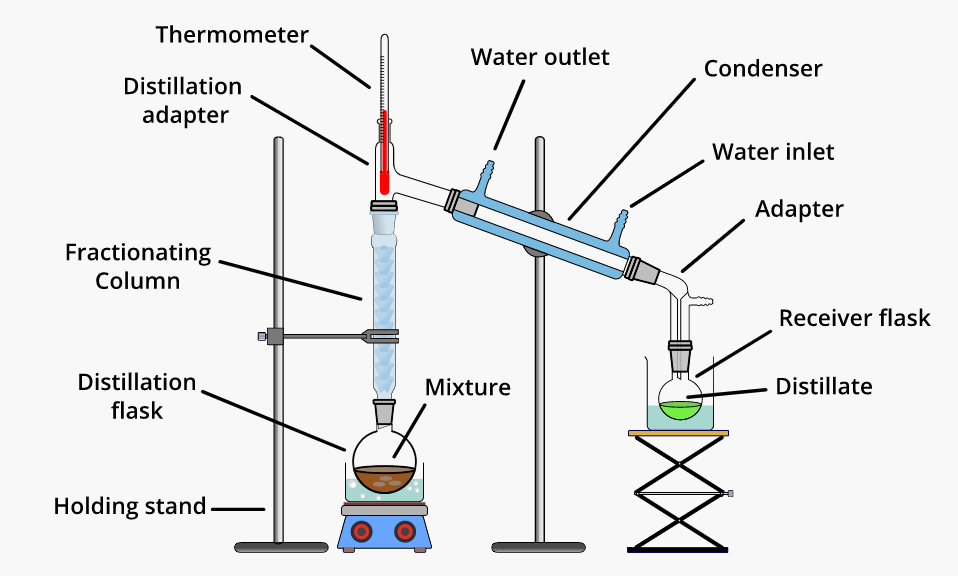
Fractional distillation is used for separating liquid mixtures with similar boiling points (separated by less than 70 degrees Celsius). A mixture of liquids is heated in this distillation process, and the resultant vapors rise in a glass tube known as a “fractionating column” and get separated. The fractionating column is positioned between the flask containing the mixture and the “Y” adaptor. The fractionating column has “theoretical plates” that allow vapors to condense, re-evaporate, and condense.
One theoretical plate corresponds to one vaporization-condensation cycle. The more volatile liquids will rise to the top of the fractionating column, while the higher boiling point liquids will sink to the bottom. The vapor cools in the condenser before dripping into the collecting. Typically, a Vigreux column is utilized.
Fractional Distillation apparatus
image source: https://psiberg.com/fractional-distillation/
A procedure to carry out the fractional distillation process is given below:
• The organic matter to be purified is placed in a round bottom flask fitted with a stir bar.
• The fractional distillation equipment is set up as shown in Figure 1.
• A heating mantle/block or oil bath is used to heat the organic mixture to an appropriate temperature.
- First, the lowest-boiling compound should be separated by adjusting the temperature.
- The condensate ring ascending slowly up in the fractionating column must be observed.
- The theoretical plates should begin to rise gradually.
- If the condensate ring stops, the temperature should be slightly raised.
- • Distillation takes place once the flask reaches an appropriate temperature. The vapor begins to condense in the liquid-cooled jacketed portion of the distillation equipment.
- After the first few drops of liquid start to drip down the condenser, the temperature should remain almost constant.
- The rate of distillation must be consistent and gradual.
- If the distillation takes a long time, wrap the column in aluminum foil or glass wool.
- The temperature of the distillate drop should be observed once the lower-boiling material is finished.
• At this point, the heating element temperature can be raised to distill the next lowest-boiling compound before moving to a new, clean receiving flask.
• Repeat this process until the desired compound is achieved.
• Once the distillation process is finished, transfer the pure organic compound into a clean, labeled vial for preservation.
Things to consider while carrying out fractional distillation:
- The thermometer should be placed at the intersection of the “Y” piece.
- Cold water should flow from the bottom of the condenser to the top.
- The system should be kept under nitrogen to avoid decomposition or interactions with oxygen.
- Keck clamps must be used on joints.
- If very low-boiling compounds are being distilled, the receiving flask can be placed in an ice bath.
- Fractional Distillation of Crude Oil:
Separating the various components of crude oil is one of the frequent industrial applications of fractional distillation. Crude oil consists of gasoline, diesel, lubricating oil, kerosene, and other chemicals. Fractional distillation aids in the successful separation of these components.
The chamber is filled with crude oil and then heated by high-pressure steam. The mixture then begins to boil, producing vapor. Several compounds enter the vapor phase. The vapor phase rises in the fractionating column, which is comprised of multiple plates. The plates have holes in them that allow vapor to travel through. Typically, the temperature at the top of the fractionating column is kept low. The components with the lowest boiling points will condense at the top of the fractionating column, while the compounds with the highest boiling points will condense at the bottom.
The condensed vapor or liquid fractions are removed from the column’s sides. The liquid fractions thus collected can then be cooled by passing them through condensers.
Factors affecting fractional distillation
The rate of distillation, or the speed at which a liquid is vaporized and condensed to obtain a purified substance, is influenced by several factors which are discussed below:
I) Temperature
The distillation rate rises with increasing temperature because higher temperatures cause the liquid to vaporize faster.
II) Surface area
The surface area of the liquid exposed to the air also influences the distillation rate. A larger surface area will lead to the faster evaporation rate of the liquid and thus faster will be the distillation rate.
III) Pressure
The distillation rate is inversely proportional to the pressure under which the distillation is performed. The lower the pressure, the faster will be the distillation rate, as the boiling point of liquid decrease with decreasing pressure.
IV) Boiling point
The boiling point of the distilled liquid also influences the distillation rate. The liquid with higher boiling points will take a longer time to vaporize and distill than the liquids with lower boiling points.
V) The volume of liquid
The lower the volume of the liquid, the faster will be the distillation rate, and vice versa.
VI) Presence of impurities
The distillation rate is also affected by the presence of impurities in the liquid. The impurities present may alter the boiling point of the liquid and this requires the additional time to be vaporized and distilled.
Advantages of Fractional Distillation
Some of the advantages of fractional distillation are as follows:
I) Simple apparatus
Fractional distillation requires simple apparatus. It includes a condenser, a distillation flask, fractionating column, a thermometer, and a bunsen burner (heat source) are among. These things allow for simple fractional distillation. They are easy to handle and readily available.
II) Use of fractionating column
The fractional distillation apparatus includes a fractionating column, which facilitates the separation of two or more components of a mixture. It is positioned between the condenser and the flask to improve separation between the two liquids being distilled. They can aid in separating chemicals based on properties such as adsorption, absorption, size, and other factors.
III) Highly efficiency
Fractional distillation is more efficient than simple distillation because it produces a purer distillate. When the temperature difference between the boiling points of the two liquids is less than 40 degrees Celsius, it is more effective to separate the two components of the mixture.
The fractional distillation apparatus uses numerous simple distillation cycles to differentiate solutions with a low or high boiling point into pure components.
IV) Does not require a high boiling point difference
The fractional distillation technique does not necessitate significant variations in boiling points between the various components of the liquid. The temperature difference between the boiling points of the two compounds can be less than 40 degrees Celsius for this process. This is a very useful method for separating two liquids.
V) Better separation of the components than other distillations
Fractional distillation is a common distillation technique because it provides better separation of the components of a mixture than other types of distillation like simple distillation, steam distillation, and so on. Since the distillate’s composition is continuously changing, this process is also called an equilibrium process.
Disadvantages of Fractional Distillation
The following are some of the disadvantages of the fractional distillation process:
I) Solute and solvent cannot be separated
The solute and solvent cannot be separated by fractional distillation. The mixture, which consists of a solute dissolved in a solvent, cannot be used. The mixture must be a liquid-liquid solution that can be distilled using the fractional distillation method.
II) Requires complex and expensive equipment
For industrial purposes, the fractional distillation process necessitates complex and costly equipment such as distillation columns and reflux. They are expensive and frequently damaged by the heat.
III) Time-consuming
The fractional distillation process takes a long time since it repeats the simple distillation process numerous times until the required components are fully separated.
IV) Requires more energy
The fractional distillation procedure must be repeated several times. The separation of the components is improved by subjecting the mixture to longer vaporization-condensation cycles. Industries have an ongoing requirement to separate more than one type of component, which uses a lot of energy.
V) Operational risk
High pressures and temperatures are involved in the fractional distillation process. As a result, there is a probability of operational risk, which can be very serious. There is the danger of an explosion. As a result, it is damaging and dangerous for the individual. So it requires specific knowledge.
Applications of Fractional Distillation
The fractional distillation process is widely used in industries. Some of the major applications of fractional distillation are as follows:
- Fractional distillation can be used to produce high-purity silicon from chlorosilanes. Silicon is most commonly found in semiconductors.
- It is used to separate air into its constituents like oxygen, nitrogen, and carbon dioxide.
- Fractional distillation is used for water purification and also for separating ethanol and water.
- It is used in several industries, including oil refineries and chemical plants, to purify and separate various organic molecules.
- This method may separate crude oil “petroleum” into numerous petroleum products such as kerosene, petrol, diesel, and many more.
Differences between Steam Distillation and Fractional Distillation
The following are the differences between Steam Distillation and Fractional Distillation:
| S.N. | Steam distillation | Fractional distillation |
| 1. | The process of separating temperature-sensitive compounds from a mixture is known as steam distillation. | The method of fractional distillation is commonly used to separate the hydrocarbon components of crude oil. |
| 2. | It can be achieved through distillation, followed by condensation. | It is carried out through a series of distillations and condensations. |
| 3. | In this process, the chemical components are vaporized at a temperature lower than their boiling point. | In this process, the chemical components are vaporized at their boiling points. |
| 4. | The compound must be insoluble in water. | Compounds may or may not be miscible with the liquid. |
References
- https://www.researchgate.net/publication/318053910_Analysis_of_Various_Liquid_Components_under_Different_Temperature_and_Density_Constraints_Pertaining_To_Fractional_Distillation.
- https://byjus.com/chemistry/fractional-distillation/.
- http://www.chem.rochester.edu/notvoodoo/pages/purification.php?page=fractional_distillation.
- https://psiberg.com/fractional-distillation/.
- https://www.toppr.com
- https://www.shalom-education.com/courses/gcsechemistry/lessons/atomic-structure-and-the-periodic-table/topic/fractional-distillation-2/
