Copper is employed in a variety of sectors, including architecture, transportation, electronics, and consumer products. Minerals are concentrated in certain types of geologic formations (ore bodies), which must be mined, processed, and purified in order to increase the metal’s applicability. The process of extraction of copper from its ores and preparing copper metal or chemical compounds for use in various goods is known as copper processing.

Occurrence and Abundance of Copper
Copper may be found in a variety of minerals, including chalcocite, chalcopyrite, bornite, cuprite, malachite, and azurite. It can be found in seaweed ashes, many marine corals, human liver, and many mollusks and arthropods. Copper metal occurs naturally, although minerals such as chalcopyrite and bornite are by far the most abundant sources.
Smelting, leaching, and electrolysis are used to extract copper from these ores and minerals. Chile, Peru, and China are the three biggest copper-producing countries.
It is found in both its native and combined states. The principal copper ores are:
(i) Copper glance; Cu2S
(ii) Copper pyrites; CuFeS2
(iii) Malachite; Cu(OH)2CuCO3
(iv) Cuprite or (Rubby copper); Cu2O
(v) Azurite; 2CuCO3.Cu(OH)2
Today, chalcopyrite (CuFeS2) is the most frequent source of copper ore, accounting for around fifty percent of copper production.
Copper Mining
Mining is the initial phase in the extraction process. Copper ore must be extracted using either open-pit or subterranean techniques.
- Open-pit mining is the most efficient technique for extracting huge quantities of material from low-grade resources close to the surface.
- Huge track-mounted drills prepare the ore for blasting, and the fragmented ore is transported to the ore-dressing facility by truck (up to 150 tons per load) or conveyor.
- In underground mining, vertical shafts are sunk more than 1,000 meters (3,300 feet) below the surface, and tunnels are constructed to reach the ore body.
- The blasted and drilled ore is lifted through the shaft and transported to the processing facility.
- In many instances, initial crushing occurs underground, but in others, a ramp and trucks transport ore to the surface.
Extraction of Copper
Copper is extracted from its ore by a series of procedures, including mining, crushing, heating, chemically isolating impurities, refining, and purifying. Creating useful items from raw resources like copper ore requires a lengthy process but is possible with careful preparation and attention to detail. Understanding how this adaptable metal is harvested will help us comprehend exactly how significant this resource is, given its wide range of historical and contemporary use.
Typically, there are three key phases involved in the extraction of copper from ore. The first phase, mineral processing, is to release the copper minerals and remove waste elements, such as alumina, limestone, pyrite, and silica, in order to concentrate the copper minerals and other nonferrous minerals of value into a product containing between 20 and 30 percent copper.
The second phase, which may involve smelting or leaching, eliminates a significant amount of impurities components, particularly iron and sulfur in the case of sulfide ores. The final process, refining, eliminates all traces of impurities and yields a copper product with a purity of 99.99 percent.
Concentration of Ores
In the ore-dressing plant, mined material is crushed in multiple stages and finely milled to release copper minerals from waste material, called gangue. In situations where the next stage is leaching (most often with oxide ores), total liberation of the copper minerals is not necessarily required; the ore just has to be crushed and ground to the amount sufficient to expose the surface of the minerals to the leaching agent. In contrast, for sulfide ores, selective flotation often follows crushing and grinding and needs an appropriate level of release.
The ore is ground into a fine powder and then suspended in water. Included in this are Collectors and Froth Stabilizers. Collectors (pine oils, fatty acids, etc.) enhance the non-wettability of the metal portion of the ore, allowing it to form froth, whereas Froth Stabilizers (cresols, aniline, etc.) maintain the froth. The oil lubricates the metal, whereas water lubricates the gangue. Paddles and air continuously agitate the solution to produce foam. This foamy metal is scraped off the surface and dried in order to reclaim it.
Froth Flotation Process
Froth flotation is an essential concentration procedure that may be used to selectively extract hydrophobic minerals from hydrophilic waste gangue. In a more straightforward setting, froth flotation is one of the most often used operational procedures for mineral beneficiation. In ore/mineral beneficiation, froth flotation is a process in which commercially valuable minerals are separated from contaminants and other minerals by being collected on the surface of a froth layer.
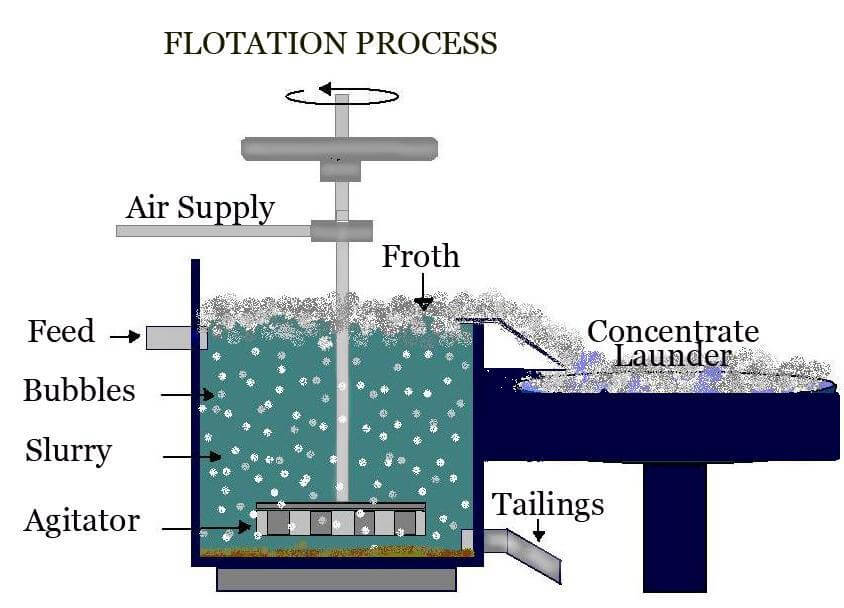
Image Source: Metallurgist
In the flotation process, finely powdered ore is agitated by mechanical and pneumatic equipment together with water and specific reagents. They generate air bubbles in the ore-water slurry combination. The reagents create an attraction between the copper mineral surface and the air bubbles. As the bubbles rise to the surface, they transport the copper particles with them, leaving the gangue minerals to be thrown as tailings.
Copper concentrate is produced by collecting the froth at the surface of the flotation cell. To maximize copper recovery and decrease losses, tailings are routinely reground and subjected to a second flotation, with the resulting concentrate added to the initial output. The flotation concentrate is subsequently dewatered and filtered to generate filter cake, which is sent to a copper smelter.
Roasting
Often, reverberating furnaces are utilized during the roasting procedure. The copper concentrate is partly oxidized in the roaster to form “calcine.” Sulfur dioxide gas is released. The reaction’s stoichiometry is as follows:
CuFeS2 + 3 O2 → 2 FeO + 2 CuS + 2 SO2
Roasting usually leaves more sulfur in the calcined product than a sinter plant does in the sintered result.
Since 2005, roasting is no longer often used in copper concentrate treatment since its combination with reverberant furnaces is not energy efficient and the SO2 concentration in the roaster’s offgas is too dilute for cost-effective capture. Direct smelting is now preferred and employs the following technologies: flash smelting, Isasmelt, Noranda, Mitsubishi, and El Teniente furnaces.
Smelting
Generally, the first melting of the material to be smelted is known as the smelting or matte smelting step. It can be carried out in a range of furnaces, including the mostly outmoded blast furnaces and reverberatory furnaces, in addition to flash furnaces, Isasmelt furnaces, etc. This stage of smelting produces matte or copper matte, a copper-enriched combination of copper, iron, and sulfur.
The objective of the matte smelting step is to remove as much iron, sulfur, and gangue minerals (such as silica, magnesia, alumina, and limestone) as possible while reducing copper loss. This is accomplished by reacting iron sulfides with oxygen (in the air or oxygen-enriched air) to generate iron oxides (mostly as FeO, but also magnetite (Fe3O4)) and sulfur dioxide. Copper sulfide and iron oxide can combine, but when a substantial amount of silica is added, a distinct slag layer forms.
The matte sulfide material includes between 45 and 70 percent copper, depending on the specific procedure. Above the matte, gangue minerals and oxidized impurities, including the majority of iron, react with the flux to create a light, fluid layer of slag. A portion of the volatile pollutants, such as sulfur, are oxidized and are carried away with the process gas stream.
Depending on the nature of the concentrate, smelting can be conducted autogenously, i.e., without the need of supplementary fuel, as is necessary in reverberatory or electric-arc smelting. In addition to decreasing fuel usage, the new procedures generate relatively small amounts of gas, which, due to its high sulfur dioxide content, is ideally suited for the manufacturing of sulfuric acid. Modern smelters are designed to collect at least 90 percent of the sulfur in the feedstock.
These steps result in the following reaction:
2 CuFeS2 + 2 SiO2 + 4O2 → Cu2S + 2 FeSiO3 + 3SO2
Copper extracted by this method is combined with slag to create a material known as Matte Copper due to its matte look. Matte Copper is converted to pure metal by blasting it with air, which mostly consists of Cu2S.
Cu2S + O2 → 2Cu + SO2
SO2 escapes from the copper, causing bubbles to form and explode.
This results in the end product having a highly blistered look, thus the name Blister Copper; 98 to 99.5% pure.
Bessemerization
When the Bessemer converter is ready, the molten matte from the blast furnace is delivered into it.
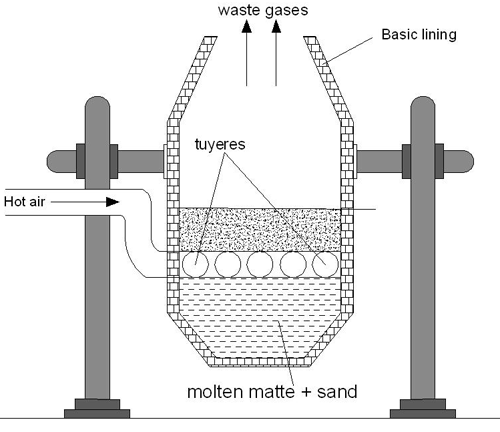
The interior of the container is coated with lime or magnesium oxide while the exterior is constructed of steel. The molten matte is hit with a hot jet of air that contains sand at the same time. When going through this procedure:
- Some of the ferrous sulfide that was present in the matte was converted to ferric oxide (FeO), which then combined with silica to produce slag.
2FeS + 3O2 → 2FeO + 2SO2
FeO + SiO2 → FeSiO3
- Copper sulfide undergoes a partial oxidation to produce cuprous oxide, which then undergoes a further reaction with any leftover copper sulfide to produce copper and sulphur dioxide.
2Cu2S + 3O2 → 2Cu2O + 2SO2
Cu2S + 2Cu2O → 6Cu + SO2
Once the reaction is finished, the converter is tipped so that the liquid copper may be poured into sand molds. These molds are then allowed to cool. As the metal is allowed to cool, sulfur dioxide, nitrogen, and oxygen are released from within it. Blistering copper is a kind of copper that has around 99% purity and was obtained in this way. The formation of blisters on the surface of the metal is where the term “blister” originates. These blisters are caused when the dissolved SO in the metal manages to escape during the solidification process.
Refining of Copper
Poling
- The blister copper is purified by heating it vigorously in a reverberant furnace in the presence of excess air.
- The contaminants are eliminated as volatile oxides or transformed into slag.
- Some copper also transforms into cuprous oxide. By stirring the molten metal with green wooden poles, this is converted back to copper.
- Cuprous oxide is converted to copper that is about 99.5% pure by the hydrocarbons found in these newly cut poles.
Electrolytic refining
This is the last phase in the pyrometallurgical processing as well as the hydrometallurgical processing.
- During the electrolytic process, copper anodes and starting sheets are submerged in an electrolytic solution that consists of copper sulfate and sulfuric acid. This solution is known as an electrolyte.
- Copper, which originates from the positively charged anode, is deposited in a pure form on the negatively charged beginning sheet, which functions as the cathode, as a result of an electric current being run through the solution.
- The anode slimes, which include trace contaminants like as precious metals, are found near the bottom of the cell and are processed further from there.
- Copper in solution that was produced by a hydrometallurgical process may be recovered in an electrolytic cell that is analogous to this one by utilizing lead as the anode. In this case, the electric current takes the copper out of solution rather than removing it from the anode so that it may be deposited on a cathode starting sheet (when the metal is plated from solution in this manner, the process is known as electrowinning).
- Both of these techniques have the ability to produce cathode copper with a purity that is greater than 99.9 percent.
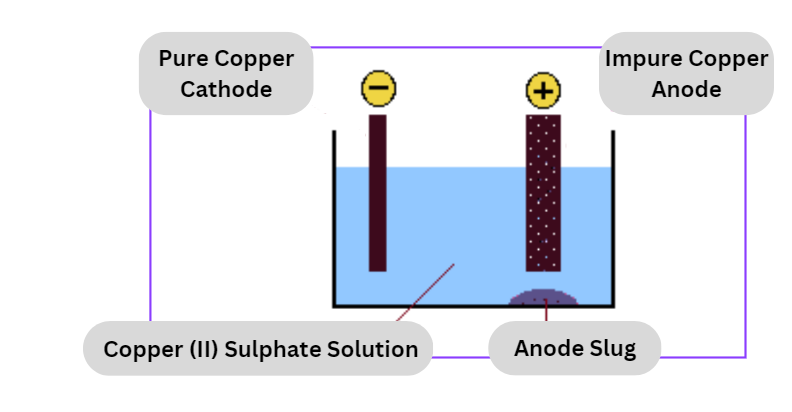
At anode: Cu – 2e¯ → Cu2+
At Cathode: Cu2+ + 2e → Cu
Zinc, nickel, iron, and other contaminants are accumulated as anode mud beneath the anode.
Long-term exposure of copper pyrites to air and precipitation produces a diluted solution of copper sulphate. The addition of scrap iron can precipitate copper from this solution. The product is then refined electrolytically.
References
- https://www.britannica.com/technology/copper-processing
- https://www.epa.gov/radiation/tenorm-copper-mining-and-production-wastes#:~:text=Copper%20is%20used%20in%20many,are%20where%20mining%20takes%20place.
- B J Elliott, J B See, and W J Rankin, “Effect of slag composition on copper losses to silica-saturated iron silicate slags,” Transactions of the Institution of Mining and Metallurgy (Section C: Mineral Processing and Extractive Metallurgy),
- W G Davenport, M King, M Schlesinger and A K Biswas, Extractive Metallurgy of Copper, Fourth Edition (Elsevier Science Limited: Kidlington, Oxford, England, 2002), 73–102.
- https://classnotes.org.in/class12/chemistry12/general-principles-processes-isolation-elements/extraction-copper-zinc/#1_Crushing_and_concentration
- T Robinson, “Electrolytic refining,” in: Extractive Metallurgy of Copper, Fourth Edition, Eds W G Davenport, M King, M Schlesinger and A K Biswas (Elsevier Science Limited: Kidlington, Oxford, England, 2002) 265–288.
