Enzymes Definition
Enzymes are biological molecules that act as biological catalysts and accelerate the rate of chemical reactions in living systems.
- Enzymes are proteins that act on molecules, known as substrates, in order to produce different molecules called products.
- Most metabolic processes in biological systems require enzymes so that the processes can occur at rates that can sustain life.
- Enzymes are composed of protein and non-protein units, which are together called holoenzymes.
- The two separate units work together to act on the substrate and produce the necessary product within the desired time.
- Enzymes work by decreasing the activation energy of a reaction, wherein the presence of enzymes, the reactions occurs up to millions of times faster.
- Enzymes are highly specific and, thus, only act on specific substrates. The specificity can be observed in the form of structural properties.
- Enzymes are not utilized during a chemical reaction and can be recovered at the end of the reaction. The enzymes also don’t affect the equilibrium of such reactions.
- Besides, enzymes also have essential industrial and medical applications as these can be used in food and pharmaceutical industries for the production of different products.
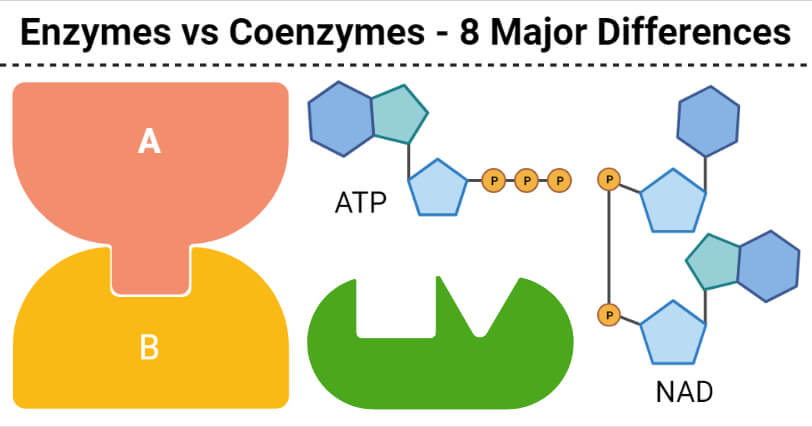
- Enzymes are usually large molecules composed of a protein unit and a coenzyme or cofactor. The protein unit without the cofactor or coenzyme is known as apoenzyme and cannot function as an enzyme.
- All enzymes have an active site that determines the specificity of the enzyme. The substrate binds itself to the active site, and the specificity of the enzyme depends on the structure of the active site.
Coenzymes Definition
Coenzymes are organic molecules that form the non-protein part of an enzyme and function as cofactors to form a complete enzyme.
- Coenzymes take part in enzyme-mediated catalysis in stoichiometric amounts like in chemical reactions.
- Coenzymes, unlike the protein part of an enzyme, get modified during the chemical reaction and might require a separate enzyme-mediated reaction in order to restore them.
- Coenzymes can be divided into two distinct groups; prosthetic groups and cosubstrates.
- Prosthetic groups are organic groups that remain covalently and permanently bonded to the protein unit of the enzyme.
- Cosubstrates, in turn, remain transiently bonded to the proteins and thus only be attached during the enzymatic process.
- Coenzymes in the living system are produced during chemical reactions and are not consumed through the diet.
- These molecules are usually thermostable and dialyzable in nature and remain either attached to the protein or in the cytoplasm of the cell.
- Coenzymes account for just 1% of the total volume of the enzyme, while the rest is occupied by the protein unit.
- The coenzymes transfer chemical groups like hydride ions, methyl groups, or acyl groups to the protein unit.
- The binding of the coenzyme to the protein unit then changes the structure of the coenzyme. The coenzymes are often also known as the second substrates.
- Coenzymes are usually smaller in size and weight when compared to the final enzymatic product.
8 Key differences (Enzymes vs Coenzymes)
| Characteristics | Enzymes | Coenzymes |
| Definition | Enzymes are biological molecules that act as biological catalysts and accelerate the rate of chemical reactions in living systems. | Coenzymes are organic molecules that form the non-protein part of an enzyme and function as cofactors to form a complete enzyme. |
| Size | Enzymes are larger in size when compared to coenzymes. | Coenzymes account for just 1% of the total volume of enzymes. |
| Nature | A complete enzyme is composed of the protein unit and non-protein organic or inorganic unit. | Coenzymes are non-protein organic compounds. |
| Function | The most important function of enzymes is to accelerate different chemical reactions. | The most important function of coenzymes is to activate enzymes by binding to the active sites. |
| Types | Enzymes can be classified into different groups based on their mode of action as transferases, hydrolases, isomerases, etc. | Coenzymes can be classified into two distinct groups; prosthetic groups and cosubstrates. |
| Changes during reaction | Enzymes remain unchanged during the chemical reaction. | Coenzymes might change in the structure during the reaction as they transfer chemical groups to other molecules. |
| Specificity | Enzymes are highly specific and thus only act on a particular group of molecules. | Coenzymes are less specific than enzymes. |
| Examples | Examples of enzymes include proteases, amylases, lipases, esterases, etc. | Examples of coenzymes include ATP, vitamins, NAD, etc. |
Examples of enzymes
Lipase
- Lipase is an enzyme that catalyzes the breakdown of lipids that are essential in various metabolic activities like digestion, transport, and processing of lipids in the living system.
- Lipases are a group of esterases that hydrolyze the ester linkage present in the lipid molecule.
- Lipases usually act at a specific position on the glycerol backbone of the substrate to produce appropriate products.
- Even though lipases are produced in the living systems by particular cells, some lipase might be expressed and secreted by pathogens during an infection.
- In the case of human lipases, most of them are produced in the digestive tract by the pancreas and liver. These have specific functions towards specific lipid molecules.
Examples of coenzymes
Thiamine pyrophosphate
- Thiamine pyrophosphate is produced from thiamine or Vitamin B1 and is involved in the formation of the enzyme thiamine diphosphokinase.
- It is an organic molecule which can be found in all living systems and catalyzes reaction involving the hydrolysis of carbohydrates.
- Thiamine pyrophosphate is synthesized within the cytoplasm, and it acts as a coenzyme for the activity of a variety of enzymes like transketolase and pyruvate- and oxoglutarate-dehydrogenases.
- The molecule also aids in the production of essential metabolic byproducts or substances like ATP, NADPH, and ribose-5-phosphate, critical for the generation of cellular energy.
- Thiamine pyrophosphate also catalyzes the reversible decarboxylation of carbon compounds.
- In people with alcohol addiction, deficiency of Vitamin B1 might act as one of the causes of Korsakoff Syndrome.
References and Sources
- Jain JL, Jain S and Jain N (2005). Fundamentals of Biochemistry. S. Chand and Company.
- Nelson DL and Cox MM. Lehninger Principles of Biochemistry. Fourth Edition.
- Berg JM et al. (2012) Biochemistry. Seventh Edition. W. H Freeman and Company
- 1% – https://www.thoughtco.com/activation-energy-example-problem-609456
- 1% – https://www.creative-enzymes.com/product/immobilized-lipase-from-pseudomonas-sp_1933.html
- 1% – https://scienceinfo.com/cofactors-vs-coenzymes/
- 1% – https://microbiologyclass.com/enzymes-definition-and-mechanism-of-action/
- 1% – https://en.wikipedia.org/wiki/Lipase
- 1% – https://en.wikipedia.org/wiki/Enyme_characteristics
- 1% – https://courses.lumenlearning.com/boundless-microbiology/chapter/organic-compounds/
- 1% – https://bristolsciencetutor.files.wordpress.com/2012/05/enzymes.pdf
- <1% – https://www.wikihow.com/Calculate-the-Concentration-of-a-Solution
- <1% – https://www.sciencedirect.com/topics/neuroscience/coenzymes
- <1% – https://www.britannica.com/science/enzyme
- <1% – https://quizlet.com/417110548/enzymes-flash-cards/
- <1% – https://journals.sagepub.com/doi/pdf/10.1177/1533210110392941
- <1% – https://en.wikipedia.org/wiki/Biocatalyst
- <1% – https://chem.libretexts.org/Courses/Athabasca_University/Chemistry_360%3A_Organic_Chemistry_II/Chapter_26%3A_Biomolecules%3A_Amino_Acids_Peptides_and_Proteins/26.10_Enzymes_and_Coenzymes
- <1% – http://attic.volgmed.ru/depts/biochem/sources/e-enzyme1.pdf

I FOUND THE INFORMATION VERY USEFUL AND WISH TO FIND MORE AND DETAILED INFORMATION OF SUCH KIND