Enzymes are biocatalysts that speed up the rate of a chemical reaction without being changed in the overall process. They consist of hundreds and thousands of amino acids liked together in a specific order. So, enzymes are proteins made up of amino acids. Enzymes mediate all cellular reactions. They have several unique properties.
- The specific enzyme catalyzes the specific reaction.
- Most of the enzymes are proteins.
- They increase the rate of reaction by lowering the activation energy. They do not affect the position of the equilibrium.
General classes
There are two general classes of the enzyme. They are as follows:
Simple enzymes
They entirely consist of amino acids. E.g., Lipase
Conjugated enzymes
They consist of a non-protein group along with the protein component. Holoenzymes are complete biologically active conjugated enzymes.
The non-protein group in conjugated is known as a cofactor, and it plays an important role in the biological activity of these enzymes. Apoenzyme is the protein component of the conjugated enzyme that is biologically inactive.
Coenzymes are organic groups that are covalently or non-covalently bonded to the protein portion of conjugated enzymes. Prosthetic groups are cofactors (either metal ions or organic molecules) that are covalently or non-covalently associated with protein.
| Enzymes or Vitamins | Cofactor | Coenzyme |
| Thiamine | – | Thiamine pyrophosphate |
| Cytochrome oxidase, xanthine, peroxidase | Fe2+ or Fe3+ | |
| Biotin | – | Biocytin |
| Pyruvate kinase | K+ |
Naming of enzymes
Many enzymes have common names, e.g., trypsin and proteolytic enzyme. They are named after the substrate or the reaction they catalyze, with the suffix -ase. For example, ATPase is the enzyme that catalyzes the breakdown of ATP, whereas ATP synthase is an enzyme that synthesizes ATP.
The common name system causes much confusion, and it provides little information about the reaction catalyzed by different enzymes. As a result, the Enzyme Commission developed a systematic naming system in which enzymes are named and classified based on the chemical reaction they catalyze.
Enzyme Commission assigned a number for each enzyme divided into four parts. E,g., EC 2.7.1.2 (hexokinase), the first three number indicates the class, sub-class, and sub-subclass, while the last number indicates the serial number in the subclass within which order the enzymes are added to the list.
Classification of enzymes
There are six different classes to which the enzymes belong. These are:
Oxidoreductase
They catalyze the oxidation-reduction reaction.

e.g.
Oxidase: uses oxygen as an electron acceptor but is not incorporated into the substrate.
Oxygenase: directly incorporates oxygen into the substrate.
Dehydrogenase uses other molecules like NAD+ as an electron acceptors.
Transferase
They involve in the transfer of groups from one molecule to another molecule.

e.g.
Transcarboxylases: transfers carboxylate group.
Kinases: transfer a phosphate group from ATP.
Phosphorylase: transfer an inorganic phosphate group.
Hydrolases
It catalyzes the reaction in which the cleavage of bonds is accomplished by adding water.

e.g.
Phosphodiesterase: cleaves the phosphodiester bond.
Peptidase: cleave peptide bonds in proteins.
Lyases
These enzymes catalyze the breaking of C-C, C-O, C-N, and other bonds by processes other than oxidation and hydrolysis.

e.g.
Aldolases: removal of aldehyde.
Synthases: link two molecules without the involvement of ATP.
Isomerases
They catalyze the intermolecular rearrangement reaction and produce the isomeric form of substrate.

e.g.
Epimerase: catalyze the inversion of asymmetric carbon atoms and the formation of epimers.
Racemases: Interconvert L and D stereoisomers.
Ligases
Catalyzes the formation of C-C, C-S, C-O, and C-N bonds by removal of the water component.

e.g.
Carboxylase: forms new bonds by introducing CO2
Succinyl CoAsynthetase: catalyzes the synthesis of succinyl CoA
Enzyme’s mode of action
During the enzymatic reaction, an enzyme incorporates the substrate into its active site and forms the enzyme-substrate complex. The complex dissociates further to yield product and enzyme. There are two models of an enzyme (receptor) and substrate (ligand) interaction.
Lock and key model
In this model, the active site of the enzyme is complementary to the shape of the substrate, and the substrate finds the correct orientation to fit in the complementary shape of the target enzyme.
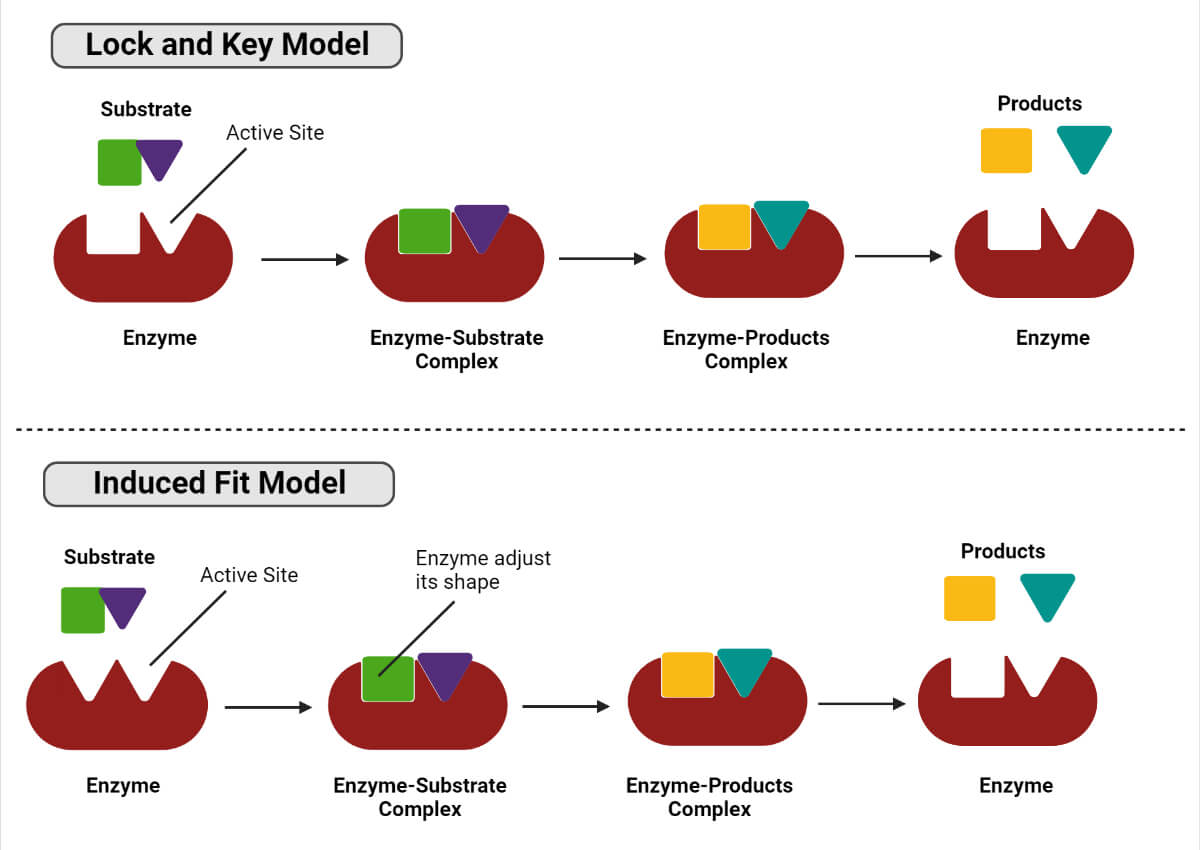
Induced fit model
In this model, the enzyme’s active site does not completely fit the substrate, so the enzyme and substrate adjust their conformation to fit together.
Catalytic strategies
Enzymes commonly use more strategies to catalyze specific reactions. They employ acid-base catalysis, metal-ion catalysis, covalent catalysis, or a combination of two strategies to catalyze various enzymatic reactions.
Covalent catalysis
It involves the formation of the irreversible covalent bond between enzyme and substrate. During covalent catalysis, the enzyme bounds to the substrate during the reaction, and after completion of the reaction, it returns to its original unmodified state.
Acid-base catalysis
This involves proton transfer. In specific acid-base catalysis, hydronium and hydroxide ions act as the acid and bases, whereas in specific acid-base catalysis donor and acceptor of the proton are other than hydronium and hydroxide ion.
Metal ion catalysis
Some enzymes require metal ions for their catalytic activity. These enzymes can be metalloenzymes or metal-activated enzymes. In the catalytic reaction, metal acts as an electrophilic catalyst stabilizing the electron density that developed during the reaction process. Metal atoms also mediate oxidation-reduction reactions.
Factors affecting the enzymatic activity
Different factors can affect the catalytic activity of enzymes. They are as follows:
Concentration of substrate
The rate of reaction increases with increasing substrate concentration up to a certain point, after which there is no significant change in the reaction rate. This is due to the substrate occupying all of the active sites of enzymes after certain concertation, leaving no space for another substrate.
Temperature
An increase in temperature increases the kinetic energy of molecules, which eventually increases the reaction rate. However, at high temperatures, the protein and enzymes become denatured, which reduces catalytic activity and, thus, the rate of reaction. Additionally, the low temperature also inactivates the enzyme. So, the enzyme-catalyzed reaction occurs at an intermediate temperature known as the optimum temperature.
pH
Enzymes are sensitive to pH due to the presence of charged amino acids at the active site. A small change in pH can change the overall charge of amino acids. So enzymes show rapid activity at their optimal pH.
Inhibitors
Inhibitors are substances that bind to the active site of the enzyme, inhibiting the binding of substrate to the active site of the enzyme. This results in a decrease in an enzyme-catalyzed reaction.
Enzyme inhibition
The inhibition enzyme activity may be reversible or irreversible depending on the inhibitors used.
Irreversible inhibition
The irreversible inhibitors covalently bind to the active site of enzymes destroying its functional group. Most of the irreversible inhibitors are toxic.
Examples of irreversible inhibitors:
Cyanide: reacts with the enzyme metal ions (i.e., Fe, Zn, Cu). Respiratory chain enzymes are the primary target
Penicillin: inhibits the enzyme responsible for bacterial cell wall synthesis.
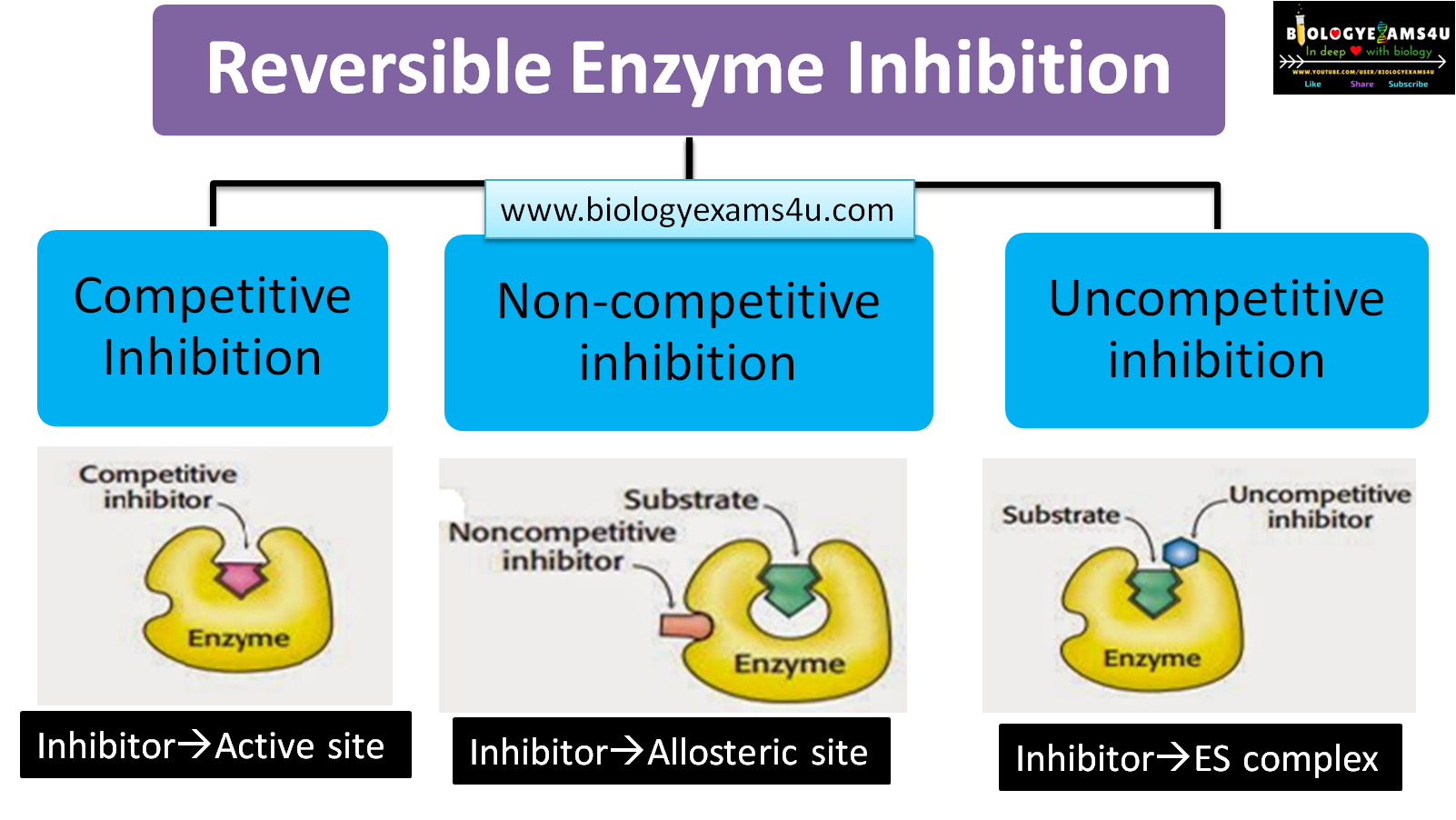
Reversible inhibition
Reversible inhibitors are attached to the enzyme through non-covalent interactions like hydrogen bonds and hydrophobic bonds and can dissociate from the enzyme. There are three types of reversible inhibition they are:
- Competitive inhibition
- Non-competitive inhibition
- Uncompetitive inhibition
Competitive inhibition
The structure of competitive inhibitors is closely related to that of the normal substrate, so it competes with the substrate for the enzyme. In this inhibition process, increasing the substrate concentration reduces the action of the inhibitor.
Non-competitive inhibition
In non-competitive inhibition, the inhibitor binds to the enzyme at a site other than the active site, i.e., the allosteric site. This interaction changes the three-dimensional configuration of the enzyme, preventing substrate binding.
Uncompetitive inhibition
The binding of inhibitors to the enzyme-substrate complex causes uncompetitive inhibition. The product leaves the active site more slowly, which slows down the reaction. It occurs most frequently in reactions involving two or more substrates.
Isozymes
Isozymes are enzymes that have different amino acid sequences but catalyze the same chemical reaction.
Zymogen
Zymogen is an inactive precursor of an enzyme. It is cleaved to form the active enzyme. Pancreatic zymogens, i.e., trypsinogen and chymotrypsinogen, are converted into the active enzyme by enteropeptidase and trypsin.
Ribozyme
Ribozyme is also called the RNA enzyme that catalyzes the chemical reaction. Although ribosomes are rare in cells, their roles are essential for life. They catalyze the cleavage of phosphodiester bonds and the synthesis of peptide bonds.
Function of enzymes
- Enzymes catalyze many cellular reactions.
- They store and release energy.
- They are involved in the growth and development of the living organism.
- They break down the nutrient into usable molecules.
- Enzymes are important in wound healing, disease diagnosis, and killing pathogenic
- microorganisms.
- They are used in many fermented products.
References
- Satyanarayana, U., & Chakrapani, U. (2013). Biochemistry. New Delhi: Elsevier Health Sciences APAC.
- https://www.britannica.com/science/enzyme
- https://byjus.com/biology/enzymes/
- https://www.toppr.com/guides/biology/biomolecules/enzymes/
- https://www.britannica.com/science/protein/The-mechanism-of-enzymatic-action
- https://www.biologyexams4u.com/2020/11/reversible-enzyme-inhibition.html
- https://pediaa.com/what-is-the-difference-between-induced-fit-and-lock-and-key/
- https://thebiologynotes.com/enzyme-inhibitors/
- https://www.khanacademy.org/science/ap-biology/cellular-energetics/environmental-impacts-on-enzyme-function/a/enzyme-regulation