An atom’s energy level is the amount of energy contained in its orbitals. An atom is typically made up of electrons that revolve in set orbits around its nucleus. They are not permitted to freely move and modify their random position while going around their orbit. This is due to their specific energy level. According to Bohr’s theory, an atom’s electrons revolve around the nucleus in certain orbits, called electron shells. Each orbit has its energy level, which is denoted by a negative value.
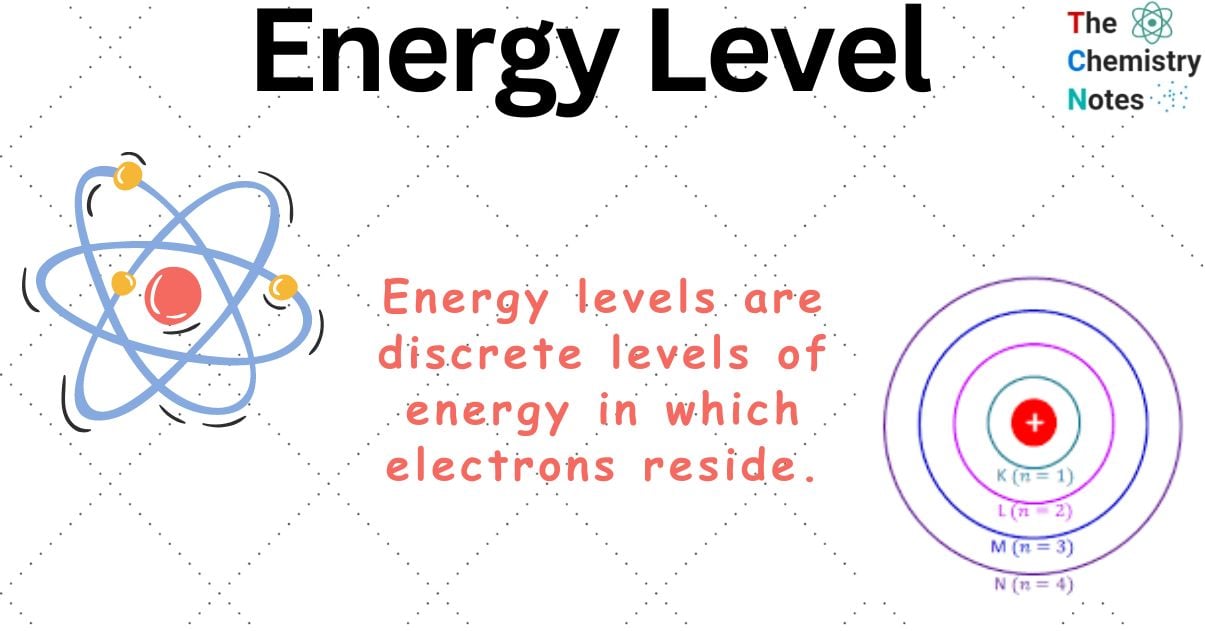
What is Energy Level?
Energy levels are discrete levels of energy in which electrons reside.
In 1913, Niels Bohr proposed his concept for energy levels, which portrays electrons circling the nucleus like planets around a star at different distances.
The energy of a level is proportional to its distance from the nucleus. When levels are close to the core of the atom, they have a low energy state, and when they are farther apart, they have a higher energy state.
- The term “ground state” refers to the lowest possible energy condition or level. Since the positively charged protons in the nucleus are attracted to the electrons, the lower energy levels will often be filled first. Lower energy electrons travel to higher energy levels, leaving vacant “slots” in higher energy states, which results in excited states.
- The term “degenerate” refers to two or more energy levels that have distinct electron configurations but the same total energy. Degenerate energy levels are what result from this.
- The energy disparities between these levels vary for various elements, allowing each one to be recognized by its distinctive spectral fingerprint.
Bohr Atomic Model
A structural representation of an atom is provided by the Bohr Model. Niels Bohr, a scientist, proposed the idea in 1913. According to this theory, electrons move in discrete circular orbits, or shells, around an atom’s nucleus. The planetary model of an atom is another name for the model.
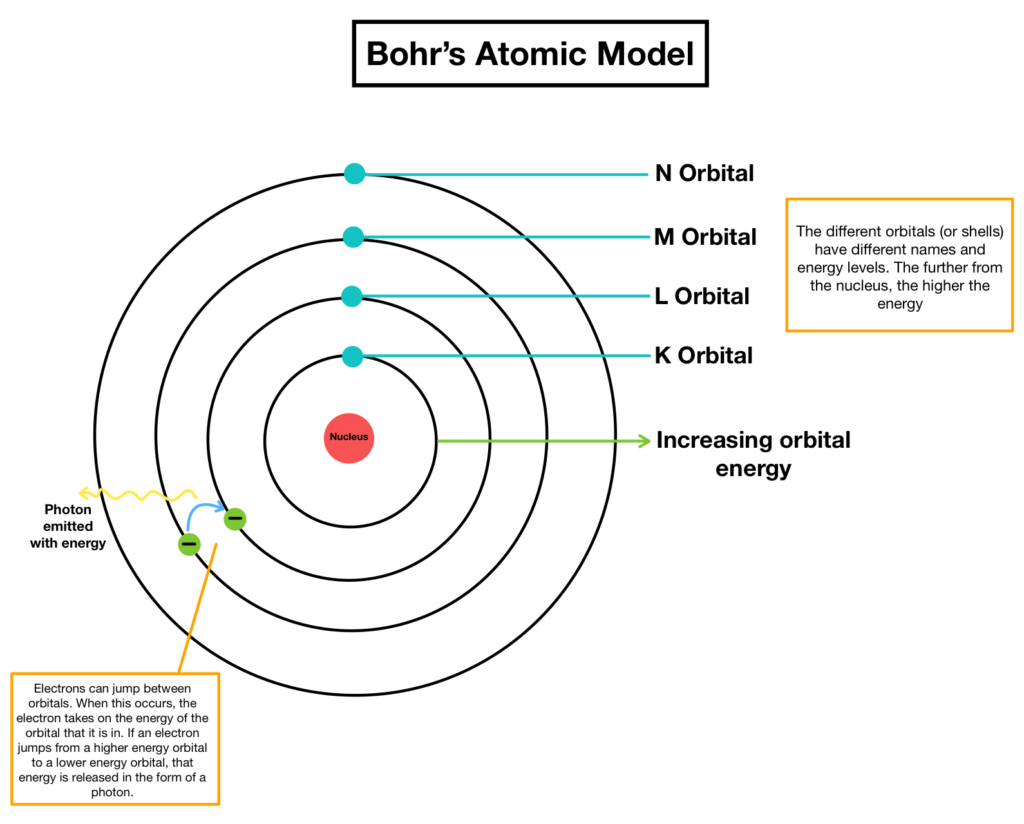
The orbits of the electrons around the nucleus resemble those of planets orbiting the sun. The electrostatic or Coulomb force between the electrons and protons keeps electrons in their orbit, just as gravity holds planets in their orbits. All of the electrons in this atomic model have to be in separate shells; they cannot be in between shells.
A unique energy level corresponds to each shell, and electrons are unable to exist outside of these orbits.
The energy of a shell decreases with increasing distance from the nucleus. An electron needs to either absorb or release energy in order to migrate to another shell. The energy differential between the shells determines how much energy is absorbed or released.
Energy Levels Explained by Bohr
- The electrons in an atom revolve around the nucleus along fixed circular routes, which are known as the energy levels, orbits, or shells of the atom.
- In their orbits, electrons don’t gain or lose energy. Bohr referred to these as energy states or energy levels since each orbit has a definite energy level.
- An electron will absorb or emit energy as it shifts to higher or lower energy levels.
- The names of the orbitals that generate the energy are 1, 2, and 4. Additionally known as K, L, M, and N shells. Primary quantum numbers (n) are the names given to these numbers.
- The K shell (n=1) with the lowest energy is the one closest to the nucleus. The higher energy levels are designated as L, M, and N, accordingly. The energy of the orbit increases with increasing separation from the nucleus.
- The maximum number of electrons that can occupy an energy level (n) is 2n2.
- When an electron transitions from one energy level to another, it absorbs the energy in the process. Energy is released when an electron transitions from a higher energy level to a lower energy level.
SubLevel
Quantum theory describes an energy level as a sublevel. Sublevels are the energies connected to electrons in chemistry. Sublevels in physics can also refer to energy related to the nucleus.
The letters s, p, d, and f stand for the various electron sublevels. Thus, for instance, the energy of electrons in the s sublevel of shell 3 differs from that of electrons in the p and d levels of shell 3. (Hydrogen is an exception to this rule. Since hydrogen only has one electron, all of its sublevels have the same energy.)
| Sublevel | Number of electrons sublevel can accommodate |
|---|---|
| s | 2 |
| p | 6 |
| d | 10 |
| f | 14 |
References
- Petrucci, Ralph, William Harwood, Geoffrey Herring, and Jeffry Madura.General Chemistry. 9th ed. Upper Saddle River, New Jersey: Pearson Prentince Hall, 2007.
- https://www.expii.com/t/bohrs-atomic-model-overview-importance-11059
- https://www.tutorialspoint.com/energy-level
- https://sciencing.com/energy-level-definition-equation-w-diagrams-13722571.html
- https://alevelchemistry.co.uk/notes/electron-configurations/
- https://www.inspiritvr.com/general-chemistry/electrons-in-atoms/energy-levels-in-chemistry-study-guide
- https://byjus.com/physics/energy-level/
- https://www.nagwa.com/en/explainers/876161937430/

