Emulsions are heterogeneous mixtures composed of two or more immiscible liquids, where one liquid is dispersed in the form of tiny or even ultramicroscopic droplets that are distributed throughout the other liquid. These structures are typically generated through the incorporation of liquid components, either in their natural state or more commonly through the implementation of various mechanisms, such as agitation. It should be noted that the fluids utilized in this process must lack any form of mutual solubility. They are deliberately created to produce a variety of products ranging from mayonnaise to everyday creams and paints. However, they are also generated as a byproduct in various industrial procedures. The stability of emulsions is of paramount importance for ensuring the quality of the end product. When encountering undesired emulsions, it is necessary to employ destabilizing agents to disrupt them.
Certain agents are known to stabilize emulsions by creating films at the surface of droplets or providing them with a form of mechanical stability. The metastable nature of emulsions leads to their eventual phase separation into two distinct liquid layers. The stability of emulsions can be disrupted through the deactivation of the emulsifying agent. This can be achieved through the introduction of suitable third-party substances or by subjecting the emulsion to thermal treatments such as freezing or heating.
What is emulsion?
An emulsion is a type of colloid in which two or more liquids that are not soluble in each other are combined, with one liquid containing dispersed droplets of the other liquid. To clarify, an emulsion is a distinct category of mixture formed by mixing two immiscible liquids. The etymology of the term “emulsion” can be traced back to its Latin root, which signifies the action of milking. This is due to the fact that milk is a classic example of an emulsion, consisting of a mixture of fat and water. Emulsification refers to the procedure of transforming a liquid mixture into an emulsion.
Emulsions belong to the broader category of colloids, which are two-phase systems of matter. While the terms colloid and emulsion are occasionally employed interchangeably, it is important to note that the term emulsion is exclusively utilized when both phases exist in a liquid state. Emulsions are commonly found in various forms such as mayonnaise, milk, lotions, and the like.
Examples of emulsions
- When oil and water are combined and agitated, the result is an emulsion. When the oil reaches the water, it will condense into droplets and spread out.
- The yolk of an egg is an emulsion that uses lecithin as an emulsifier.
- Espresso is served with a water-and-coffee-oil emulsion called crema.
- Butter is a fat-and-water emulsion.
- Silver halide in gelatin emulsion coats the film’s photosensitive side.
Properties of emulsions
- There is a sharp transition between the two phases, which is where the term “interface” comes from; this shows that emulsions are both continuous and dispersed.
- They are typically produced through the process of continuous mixing or agitation of two distinct phases.
- Particles in an emulsion always organize into highly dynamic and heterogeneous formations on a microscale.
- Extremely unstable systems like emulsions can only be stabilized with the help of an emulsifying agent or emulsifier (often surface active chemicals, also known as “surfactants”).
- In situations where emulsifying agents are absent or when the emulsion is stored for extended periods, it is common for the phases within the emulsion to separate, leading to the phenomenon known as “emulsion cracking” or “phase inversion.”
- The cloudy appearance of emulsions can be attributed to the scattering of light passing through them, which is caused by the presence of numerous phase interfaces.
- When light is dispersed in equal proportions, they exhibit a white coloration.
- When an emulsion is diluted, it causes a greater fraction of high-frequency and low-wavelength light to scatter, resulting in a blue appearance. The phenomenon under discussion is commonly known as the Tyndall effect.
- Demuslfication: Emulsions can be separated into their constituent liquids through various methods such as heating, freezing, centrifugation, or the addition of significant quantities of electrolytes. This process is commonly referred to as ‘demulsification’. The emulsifying agent is disrupted, resulting in their fragmentation. An instance of an oil-water emulsion that is stabilized by soap can be destabilized through the introduction of a potent acid. The chemical reaction involving acid catalysis results in the conversion of soap molecules into free fatty acids that are insoluble in water.
- They are capable of being diluted with any quantity of the dispersion medium. Conversely, the liquid that is dispersed will promptly separate into a distinct layer upon being combined with it. The previously mentioned characteristic of emulsions is utilized for the purpose of identifying the specific type of emulsion under consideration.
Types of emulsions
Emulsions can be categorized into two distinct types based on the characteristics of the dispersion medium and the dispersed phase.
- Oil in water (O/W): In this particular emulsion, the dispersed phase comprises oil, while the dispersion medium consists of water. Milk is widely regarded as the most excellent example of an oil-in-water (o/w) emulsion. Milk comprises of fat globules, which serve as the dispersed phase, and water, which functions as the dispersion medium. Other examples include disappearing lotion and similar products.
- Water in oil (w/o): It involves water as the dispersed phase and oil as the dispersion medium. Margarine, a commonly utilized spread for culinary purposes, is a good example of a water-in-oil emulsion. Butter and cold cream are conventional demonstrations of such types of emulsions. Some examples include cod liver oil, among others.
How emulsions are formed?
Emulsions are produced through the process of combining two liquids that are immiscible, such as oil and water, with the aid of an emulsifying agent. This agent may take the form of a protein, phospholipid, or even a nanoparticle. They can exist in two forms: oil-in-water (O/W) emulsions, where the dispersed phase is oil and the continuous phase is water, or water-in-oil (W/O) emulsions, where the phases are reversed. The type of emulsion formed is dependent on the emulsifier employed. The hydrophilic-lipophilic balance (HLB) of a surfactant is a quantitative measure of its relative hydrophilicity or lipophilicity. Surfactants that are hydrophilic in nature exhibit solubility in water and function as emulsifying agents for oil-in-water (O/W) emulsions. Conversely, surfactants that are lipophilic or hydrophobic in nature exhibit solubility in oil and function as emulsifiers for water-in-oil (W/O) emulsions.
How do emulsions break?
Four distinct mechanisms are responsible for the destabilization of emulsions, namely-
- Creaming/sedimentation
- Flocculation
- Coalescence
- Ostwald ripening
Creaming is a phenomenon that arises from the separation of an emulsion, which can be attributed to a disparity in density between the constituent components. Specifically, the relatively lighter oil droplets tend to ascend towards the uppermost layer. The process of sedimentation is analogous in nature, however, it occurs predominantly in emulsions of water-in-oil, wherein the denser water droplets tend to accumulate at the bottom of the emulsion. The process of creaming or sedimentation can be impeded by the presence of a continuous phase with high viscosity.
Flocculation refers to the process by which emulsion droplets coalesce and combine to form larger units.
Coalescence is a phenomenon in which small droplets combine with each other, resulting in the formation of a bigger droplet. This phenomenon takes place when liquid droplets make contact with one another, resulting in the rupture of the interfacial film. The phenomenon of phase separation will occur in the course of time.
Ostwald ripening is a phenomenon whereby smaller droplets dissolve in the continuous phase and subsequently deposit onto larger droplets in order to attain a thermodynamically more stable state.
Difference between colloid and emulsion
Occasionally, the terms “colloid” and “emulsion” can be used synonymously; however, the term emulsion is specifically applicable when both phases of a mixture are in a liquid state. The constituents of a colloid are capable of existing in any of the three phases of matter. An emulsion can be classified as a specific type of colloid, however, it is important to note that not all colloids can be categorized as emulsions.
Emulsifying agents
Surface active agents are utilized in emulsions to stabilize the two phases. The substance exerts an effect on the interface, leading to an increase in the kinetic stability of an emulsion. This results in the preservation of the droplet size over time, thereby promoting the stabilization of the emulsion.
Emulsifiers are chemical substances that commonly possess a hydrophilic or polar component that is soluble in water, as well as a hydrophobic or lipophilic component that is non-polar. Emulsifiers exhibit varying degrees of solubility in either aqueous or oily media.
As per Bancroft’s rule, emulsifiers and emulsifying particles have a tendency to facilitate the dispersion of the phase in which they exhibit poor solubility. Proteins exhibit a higher solubility in water as compared to oil, thereby facilitating the formation of oil-in-water emulsions. This phenomenon is characterized by the dispersion of oil droplets throughout a continuous aqueous phase, which is attributed to the emulsifying properties of proteins. Emulsifiers with higher solubility in water tend to produce emulsions of oil-in-water type, whereas emulsifiers with higher solubility in oil tend to produce emulsions of water-in-oil type. Some instances of food additives are egg yolk, sodium phosphates, and sodium stearoyl lactylate.
Role of emulsifier
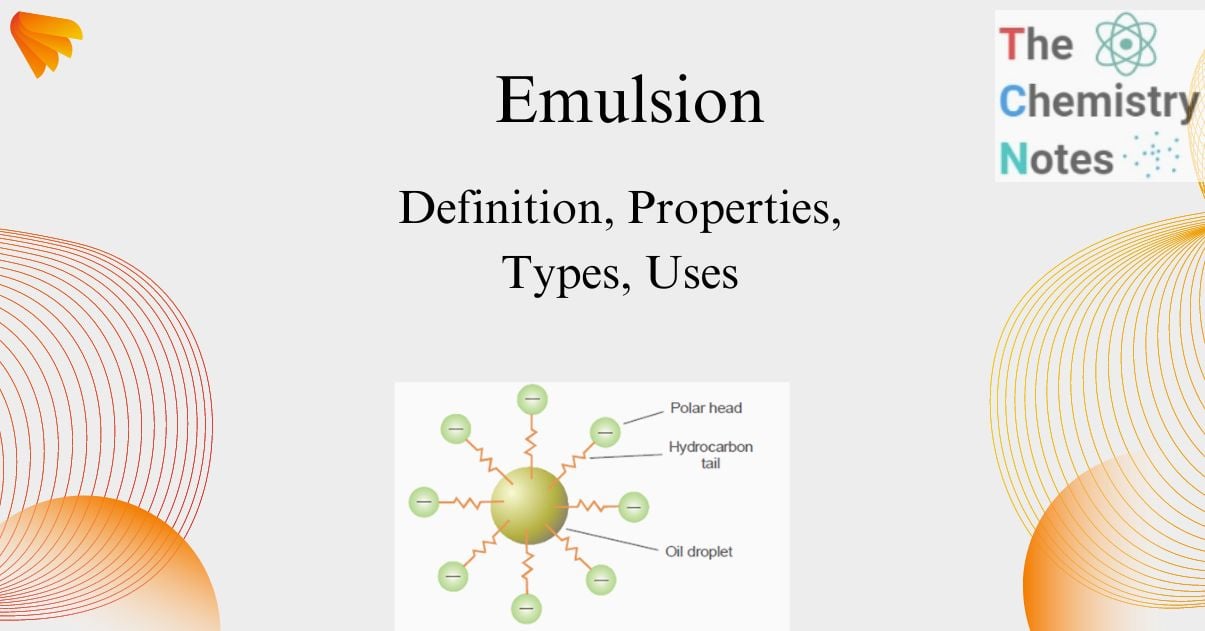
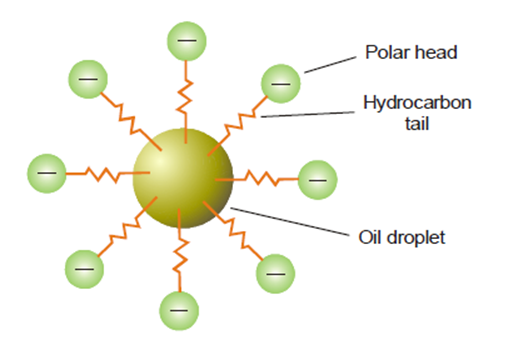
The emulsifier exhibits a concentration gradient at the interface, thereby mitigating the surface tension on one side of the immiscible liquids, leading to the formation of droplets. Soap is composed of a lengthy hydrocarbon tail that is oil-soluble, and a polar head consisting of -COO–Na+ that is water-soluble. The O/W type emulsion is characterized by the insertion of the tail into the oil droplet, with the head extending into the aqueous phase. Therefore, the soap functions as an intermediary, preventing the coalescence of emulsified droplets.
How does emulsification work?
The process of emulsification may involve a variety of distinct chemical and physical processes and mechanisms as discussed below:
- Surface tension theory: The phenomenon of emulsification can be observed when the interfacial surface tension between two liquids is lowered. This is the mechanism by which surfactants operate.
- Viscosity modification: Specific emulsifiers enhance the viscosity of the medium, facilitating the sustained suspension of globules. Several substances are utilized in this context, such as acacia and tragacanth hydrocolloids, glycerine, and the polymer carboxymethyl cellulose.
- Repulsion theory: According to the theory, the emulsifying agent generates a layer on a particular phase that gives rise to droplets, which exhibit mutual repulsion. The repulsive force acting upon them results in their suspension within the dispersion medium.
Identification of the types of emulsions
- Dilution test: When water is introduced to an o/w emulsion, the emulsion will maintain its stability due to water serving as the dispersion medium. However, the addition of oil to the emulsion will result in its destabilization, as oil and water are immiscible. Likewise, an emulsion lacking emulsifiers can be thinned with oil and maintain stability, but the introduction of water would result in destabilization.
- Conductivity test: The experimental setup involves the placement of the emulsion between two electrodes, with a circuit connected to a bulb. An oil-in-water emulsion exhibits electrical conductivity similar to that of water, whereas a water-in-oil emulsion does not demonstrate electrical conductivity.
- Dye test: A water-soluble dye is introduced into the emulsion. In the case of an oil-in-water emulsion, the dispersion medium exhibits a red hue while the dispersed phase remains colorless, and conversely, in a water-in-oil emulsion, the dispersion medium is colorless while the dispersed phase displays a red coloration.
Uses of emulsions
- Oil-in-water emulsions are frequently found in various food items. Examples represent butter, margarine, homogenized milk, mayonnaise, and so on.
- It plays a crucial role in the production of polymer dispersions. These comprise fundamental constituents of adhesives and coatings.
- They are a common form of cosmetic and pharmaceutical dosage forms. Emulsions, such as lotions, creams, and biphasic makeup removers, are commonly used in the cosmetic industry and are a common form of both oral and topical dosage forms. Microemulsions have been employed for the purpose of administering vaccines and eradicating microorganisms.
- This is employed in the context of fire suppression.
- Commonly employed in the fields of cosmetics, pharmaceuticals, and personal hygiene.
References
- https://www.toppr.com/guides/chemistry/surface-chemistry/emulsions/
- https://www.vedantu.com/iit-jee/emulsion
- https://www.biolinscientific.com/blog/how-emulsions-form-and-break
- https://www.thoughtco.com/definition-of-emulsion-605086
