Interesting Science Videos
Elements Definition
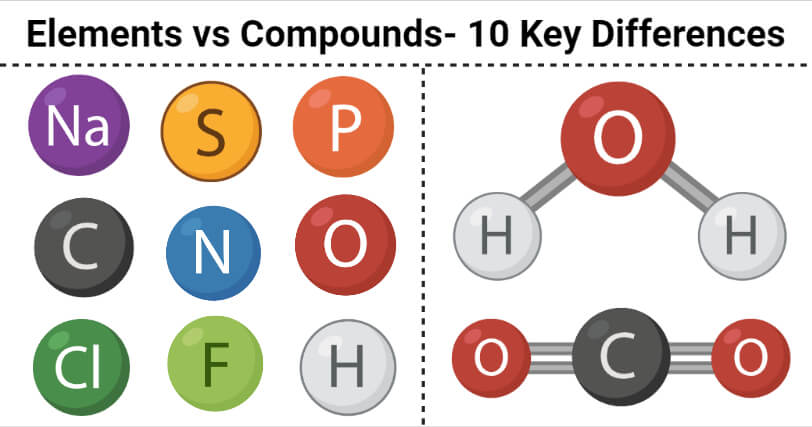
Elements are pure substances that consist of a single type of atoms and cannot be broken down into smaller units by chemical means.
- Elements are basic chemical species that combine with other elements to form complex species like compounds and molecules.
- Elements are defined by the number of protons in the nucleus, which is referred to as its atomic number.
- The number of proteins also determines the electric charge of the element as it determines the number of electrons in a non-ionized state.
- Different physical and chemical characteristics of chemical substances are defined by the type of elements present in them.
- There are 118 elements known till far, out of which 94 are naturally occurring and can be found in different forms.
- The naturally occurring elements are made as a result of various natural methods of nucleosynthesis.
- Elements are placed in different groups in a periodic table as metals, nonmetals, and metalloids based on their properties.
- Most elements exist in a solid-state at conventional temperatures and atmospheric pressure. Specific elements like bromine and mercury are in a liquid state, while several elements exist in the gaseous state.
- Elements are provided with names and are represented with symbols and numbers.
- Elements are essential as these are required for the formation of compounds and larger substances, which then make up all the matter on earth.
- Various elements like iron, potassium, and zinc are present as mineral and micronutrients in living systems.
- Some of the examples of elements include oxygen, nitrogen, iron, gold, etc.
Compounds Definition
Compounds are chemical substances that are made up of two or more atoms of the same or different elements that are linked together by chemical linkages.
- Compounds are defined by the type of elements present in them and the type of chemical bonding. Compounds, in general, can be ionic, covalent, or metallic depending on the type of bond. The bonding also determines the physical and chemical properties of compounds.
- Compounds can be formed as a result of a chemical reaction between other compounds or by the combination of atoms of the same or different elements.
- The formation of new compounds by the reaction between two existing compounds is the result of the breaking of old bonds and the formation of new ones.
- There are more than 3000 compounds in the world that are registered for use, which are produced by various combinations of the known elements.
- Compounds usually have a specific stoichiometric proportion, and the proportion is the same in a compound irrespective of its source.
- Compounds are represented by chemical formulas which provide information on the type and number of atoms present in the compounds.
- Compounds can be broken down into elements by different chemical and physical means.
- Compounds are considered impure substances as they are composed of more than one element.
- Different compounds are represented by chemical formulas, which include the symbols of the element and their proportion.
- Chemical compounds are also defined by chemical structures held together by the arrangement of chemical bonds. Depending on the type of bond holding different elements together in a compound, these have different structures.
- Some common examples of compounds include water, carbon dioxide, ammonia, magnesium sulfate, etc.
10 Major Differences (Elements vs Compounds)
| Characteristics | Elements | Compounds |
| Definition | Elements are pure substances that consist of a single type of atoms and cannot be broken down into smaller units by chemical means. | Compounds are chemical substances that are made up of two or more atoms of the same or different elements that are linked together by chemical linkages. |
| Nature | Elements are considered pure substances. | Compounds are considered impure substances. |
| Number | There are 118 known elements in the world. | There are more than 3000 different compounds that are registered for use. |
| Types | Elements can be divided into metals, nonmetals, and metalloids. | Compounds can be divided on the basis of the type of chemical bonds as covalent, metallic, and ionic compounds. |
| Nomenclature | Elements are named with symbols and numbers. | Compounds are represented by their chemical formula. |
| Made up of | Elements are made up of the same type of atoms. | Compounds are made up of molecules that consist of atoms of different elements. |
| Breakdown | Elements cannot be broken down into smaller units by chemical reactions. | Compounds can be broken down into simpler substances by chemical reactions. |
| Properties | The chemical and physical properties of elements are defined by the atoms present in the elements. | The chemical and physical properties of compounds are defined by the type of elements and the type of chemical bonding in the compound. |
| Composition | Elements contain atoms that define the properties of the substance. | Compounds are composed of elements that are present in a definite proportion. |
| Examples | Examples of elements include oxygen, nitrogen, iron, gold, etc. | Examples of compounds include water, carbon dioxide, ammonia, magnesium sulfate, etc. |
Examples of Elements
Oxygen
- Oxygen is a chemical element that is a highly reactive nonmetal with atomic number 8 and the symbol O. It is the third most abundant element in the universe.
- It is an oxidizing agent that can form oxides with multiple elements as well as compounds.
- Oxygen is a colorless and odorless gas that is essential to living beings as it is required for different biochemical activities in the body. It is also the second most abundant gas in the atmosphere.
- It was discovered in 1772 by a Swedish chemist, Carl Wilhelm Scheele, during his studies on potassium nitrate and mercuric oxides.
- The nucleus of the oxygen atom consists of eight protons and neutrons. The nucleus is surrounded by eight electrons rotating in different orbits.
- Oxygen on earth is replenished by photosynthesis which produces oxygen in the presence of sunlight, carbon dioxide, and water.
- In living systems, oxygen acts as a major constituent in the form of water. Water is the most important compound of oxygen.
Examples of Compounds
Ammonia
- Ammonia is composed of nitrogen and hydrogen in the ratio of 1:3. The molecular formula of the compound is NH3.
- Ammonia in nature occurs as common nitrogenous waste, particularly among aquatic animals.
- It is used to provide nutritional needs to terrestrial organisms as it serves as a precursor to food and fertilizers.
- At room temperature, ammonia exists as a colorless gas with a characteristic pungent smell.
- It can, however, be easily liquefied due to the presence of strong hydrogen bonding between the molecules.
- The ammonia molecule exists in a trigonal pyramidal shape with a bond angle of 106.7°. The molecule is polar as it forms a hydrogen bond with other ammonia molecules.
References and Sources
- Gautum SD, Pant M and Adhikari NR (2016). Comprehensive Chemistry, Part 2. Sixth Edition. Heritage Publishers and Distributors Pvt. Ltd
- 2% – https://www.nuclear-power.net/Oxygen-properties/
- 2% – https://www.differencebetween.com/difference-between-element-and-vs-molecule/
- 2% – https://en.wikipedia.org/wiki/Liquid_ammonia
- 1% – https://www.thoughtco.com/protons-neutrons-and-electrons-in-an-atom-603818
- 1% – https://www.quora.com/Are-there-more-elements-than-compounds
- 1% – https://www.enotes.com/homework-help/what-are-some-examples-of-compounds-131353
- 1% – https://www.chemicool.com/definition/compound.html
- 1% – https://www.austincc.edu/lesalbin/CHEMISTRY%20REVIEW.htm
- 1% – https://www.ausetute.com.au/elements.html
- 1% – https://vivadifferences.com/difference-between-elements-and-compounds-with-example/
- 1% – https://quizlet.com/27719928/elements-compounds-mixtures-flash-cards/
- 1% – https://quizlet.com/185890/chapter-1-chemical-reactions-flash-cards/
- 1% – https://quizizz.com/admin/quiz/57fd8ae84b7392052688b62b/elements-and-compounds
- 1% – https://infogalactic.com/info/Chemical_element
- 1% – https://en.wikipedia.org/wiki/Hydrogen_bond
- 1% – https://en.m.wikipedia.org/wiki/Active_oxygen
- 1% – https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_Chemistry_-_The_Central_Science_(Brown_et_al.)/07._Periodic_Properties_of_the_Elements/7.6%3A_Metals_Nonmetals_and_Metalloids
- 1% – https://brainly.com/question/14643251
- 1% – https://brainly.com/question/10473791
- 1% – http://www.ipsl.co.tt/HTMLgeneral/Products2.htm
- <1% – https://www.britannica.com/science/hydride
- <1% – https://simple.wikipedia.org/wiki/Atom
- <1% – https://sciencing.com/photosynthesis-important-organisms-6389083.html
- <1% – https://chemed.chem.purdue.edu/genchem/topicreview/bp/ch2/index.php

Point 3 in first section has a spelling error