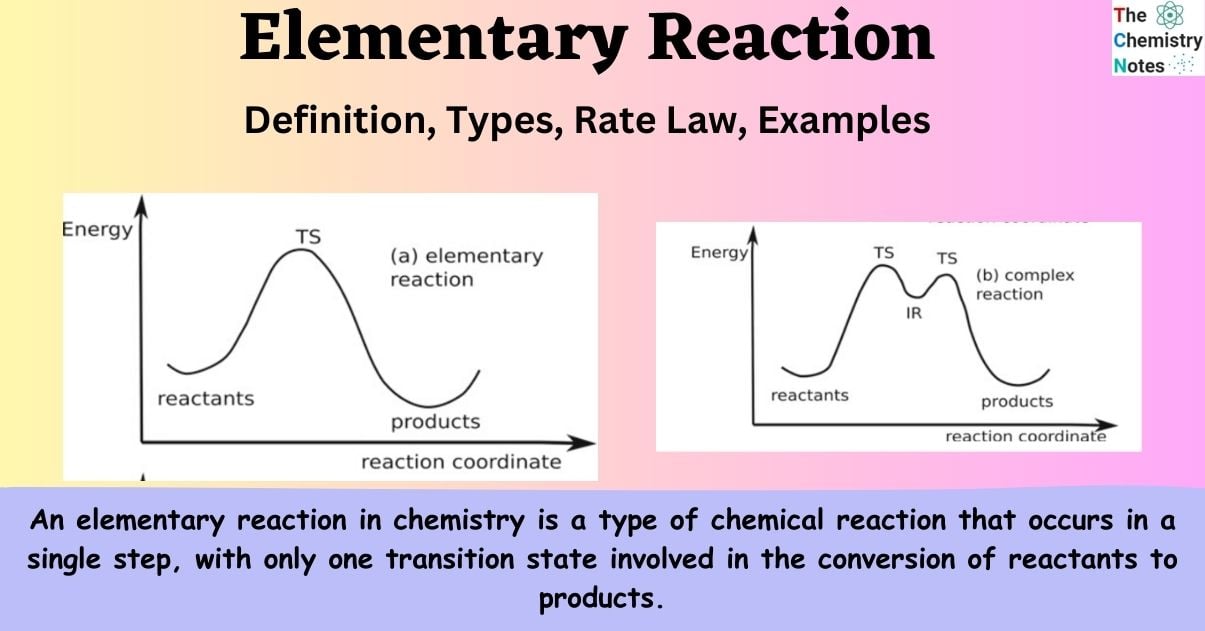
An elementary reaction in chemistry is a type of chemical reaction that occurs in a single step, with only one transition state involved in the conversion of reactants to products. An elementary reaction lacks the ability to decompose into simpler reactions and typically does not involve any intermediate steps. A complex reaction, also known as a non-elementary or composite reaction, is composed of several elementary reactions. These reactions involve intermediates and multiple transition states, leading from the reactants to the intermediates and finally to the products.
What is an Elementary Reaction?
An elementary reaction is a type of chemical reaction in which the reactants directly form products in a single step, with the involvement of only one transition state. No intermediate products are formed here.
An elementary reaction is a chemical reaction where the products are directly formed from the reactants. On the other hand, a non-elementary or complex reaction is characterized by the formation of intermediates that subsequently lead to the production of the final products.
An elementary reaction involves the collision, breaking apart, or rearrangement of atomic-scale particles to form reaction products and/or intermediates. The equation of an elementary reaction precisely indicates the atoms or molecules participating in the reaction.
Some examples of elementary reactions are cis-trans isomerization, thermal decomposition, and nucleophilic substitution.
Reaction Order and Molecularity of Reactions
The reaction order is an empirical quantity that is determined from the rate law obtained through experiments.
Molecularity refers to the number of molecules that participate in an elementary reaction proposed as a distinct step in a mechanism.
The molecularity of an elementary reaction is equivalent to its order. The rate law cannot be determined from the balanced chemical equation for the overall reaction, unless it is a single step mechanism and is therefore also an elementary step.
Examples of Elementary Reactions
- Cis-trans isomerization
- Racemization
- Thermal decomposition reactions
- Ring opening reactions
- Radioactive decay
- Nucleophilic substitution
Types of Elementary Reactions
Unimolecular Reaction
A unimolecular reaction refers to a chemical process in which a single molecule undergoes rearrangement, resulting in the formation of one or more products.
A → products
For Examples: radioactive decay, cis-trans isomerization, racemization, ring opening, thermal decomposition
Bimolecular Reaction
A bimolecular reaction occurs when two particles collide and result in the formation of one or more products. Bimolecular reactions are classified as second-order reactions, meaning that the rate of the chemical reaction is directly proportional to the concentration of the two chemical species that are involved in the reaction as reactants. This reaction is frequently observed in the field of organic chemistry.
A + A → products
A + B → products
For Example: nucleophilic substitution
Termolecular Reaction
A termolecular reaction occurs when three particles collide simultaneously and undergo a chemical reaction. Termolecular reactions are rare because it is unlikely for three reactants to collide simultaneously under the appropriate conditions to produce a chemical reaction.
A + A + A → products
A + A + B → products
A + B + C → products
Rate Law for Elementary Reactions
- Unimolecular Reaction
A → products
Rate Law: Rate = k[A]: Where [A] is the concentration of A in moles per liter.
Example: The gas-phase decomposition of cyclobutane (C4H8) to ethylene (C2H4) occurs via a unimolecular, single-step mechanism.
C4H8 → 2 C2H4
∴ Rate = k [C4H8]
- Bimolecular Reaction
A + A → Products Rate = k[A]2
A + B → Products Rate = k[A][B]
Example: The reaction between nitrogen dioxide (NO2) and carbon monoxide (CO) gives nitric oxide (NO) and carbon dioxide (CO2).
NO2(g) + CO(g) → NO(g) + CO2(g)
∴ Rate = k [NO2][CO]
- Termolecular Reaction
2A + B → products Rate = k[A]2[B]
A + B + C → products Rate = k[A][B][C]
Example: The reaction of nitric oxide (NO) with chlorine (Cl) gives nitrosyl chloride (NOCl).
2NO + Cl2 → 2NOCl
∴ Rate = k [NO]2[Cl2]
Consecutive elementary reactions
Consecutive elementary reactions refer to a series of chemical reactions that occur one after the other, where the product of the first reaction becomes the reactant of the second reaction, and so on. Each reaction in the series is an elementary reaction, meaning it occurs in a single step and involves only a small number of molecules.
Some reactions involve the formation of an intermediate (I) as part of the process. One example of an irreversible reaction is:
A→I→P
The intermediate(I) is present in the reaction steps but does not appear in the overall reaction: A→P.
In order to explain consecutive reactions let us consider a consecutive occurrence of two first order reversible reactions
A→B→C
The concentrations of these substances can be calculated by integrating the system of two kinetic equations. These calculations demonstrate that the concentration of B initially rises during the first reaction, but subsequently decreases as it is consumed in the second first-order reaction.
When reactions are reversible, they can lead to more complex consecutive reactions. As a result, calculating the concentration of the intermediate becomes more complicated.
Example of consecutive elementary reaction
Some of the examples of consecutive reactions are polymerisation, thermal cracking and chlorination of hydrocarbons.
-Chlorination of hydrocarbons steps are
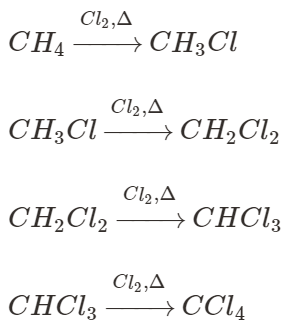
Based on the equations presented, it is understood that the chlorination of methane takes place in four distinct steps. Chloromethane is produced when methane is reacted with chlorine in the presence of light. When chloromethane is exposed to light and reacted with chlorine, it produces dichloromethane. The same step is being repeated: first, we obtain chloroform, and then we obtain carbon tetrachloride once again.
Consecutive reactions are often mistaken for parallel reactions. Parallel reaction refers to the occurrence of two reactions simultaneously, resulting in the formation of two or more products.
Elementary Reaction in Terms of Energy
Although a reaction may be highly exothermic, indicating a strong thermodynamic preference for products over reactants, there may still be considerable energy barriers that prevent the formation of products. A reaction is typically triggered by the breaking of a specific chemical bond, which necessitates the input of energy to take place.
Certain reactions, such as radical-radical or ion-molecule reactions, exhibit a negligible activation barrier for the reaction to occur. Nevertheless, there could still be minor energetic obstacles related to other processes implicated in the reaction. There are various obstacles that hinder the reaction process. These obstacles may arise from the reactants diffusing together through the gas-phase or through solution.
Additionally, there are “centrifugal barriers” which are more uncommon and are associated with the conservation of angular momentum that results from the orbital motion of the reactants in the gas phase. Taking into account these factors, we can conceptualize an elementary reaction as a conversion from one atomic or molecular state to another, which is separated by an energy barrier, in terms of energy.
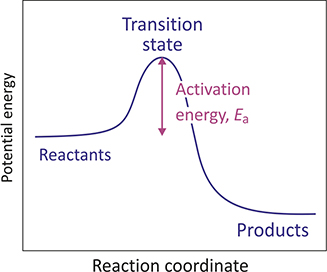
The barrier that affects the rate of a reaction at a specific temperature is known by different names such as potential barrier, activation barrier, or activation energy. If the barrier is low, the thermal energy possessed by the reactants will typically be sufficient to overcome the barrier and transition to the products, resulting in a rapid reaction.
If the barrier is high, only a small proportion of the reactants will possess enough energy, resulting in a significantly slower reaction. Raising the temperature of the reaction mixture will result in a higher proportion of reactants having enough energy to overcome the activation barrier and initiate the reaction, leading to an increase in the rate of the reaction.
Difference Between Elementary and Non Elementary Reaction
The distinction between elementary and non-elementary reactions is based on the quantity of sub-steps involved in the chemical reaction.
- The primary distinction between elementary and non-elementary reactions is that the former occurs in a single step, whereas the latter occurs in multiple steps.
- In elementary reactions, a single transition state exists and no detectable intermediates are produced during the reaction. In non-elementary reactions, there exists a sequence of transition states that involve multiple intermediates, which can be readily detected.
- Elementary reactions are considered to be simple, whereas non-elementary reactions are considered to be complex.
- The order of a reaction in an elementary reaction is equivalent to the stoichiometric coefficients of the reaction. However, in a non-elementary reaction, the order of the reaction may or may not be the same as the stoichiometric coefficients of the reaction.
| Molecularity | Elementary Step | Rate Law | Example |
|---|---|---|---|
| Unimolecular | A → Products | rate = k[A] | N2O4(g) → 2NO2(g) |
| Bimolecular | A + A → Products | rate = k[A]2 | 2NOCl → 2NO(g) + Cl2(g) |
| Biomolecular | A + B → Products | rate = k[A][B] | CO(g) + NO3(g) → NO2(g) + CO2(g) |
| Termolecular | A + A + B → Products | rate = k[A]2[B] | 2NO(g) + O2(g) → 2NO2(g) |
| Termolecular | A + B + C → Products | rate = k[A][B][C] | O(g) + O2(g) + M → O3(g) + M |
Video on Rate law of Elementary Reaction
References
- Helmenstine, Anne Marie, Ph.D. (2023, April 5). Elementary Reaction Definition. Retrieved from https://www.thoughtco.com/definition-of-elementary-reaction-605078
- https://www.coursehero.com/study-guides/introchem/rate-laws-for-elementary-steps/
- https://iopscience.iop.org/book/mono/978-1-6817-4664-7/chapter/bk978-1-6817-4664-7ch1
- https://staff.um.edu.mt/jgri1/teaching/che2372/notes/08/01/elementary_rxns.html
- Chang, Raymond. “Chemical Kinetics.” Physical Chemistry for the Biosciences. Sansalito, CA: University Science, 2005. 325-328. Print.
- Olbregts, J. (1985), Termolecular reaction of nitrogen monoxide and oxygen: A still unsolved problem. International Journal of Chemical Kinetics, 17: 835–848. doi: 10.1002/kin.550170805.
- Kerr, James Alistair. “Notes on the Tables.” CRC Handbook of bimolecular and termolelucar gas reactions . Boca Raton, Florida: CRC Press, 1987. 2. Print.
- https://www.chemistrylearner.com/elementary-reaction.html
- https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Kinetics/03%3A_Rate_Laws/3.02%3A_Reaction_Mechanisms/3.2.01%3A_Elementary_Reactions
- https://www.differencebetween.com/difference-between-elementary-and-non-elementary-reaction/
- Baer, Tomas, and William L. Hase. “Introduction.” Unimolecular reaction dynamics: theory and experiments. New York: Oxford University Press, 1996. 4-5. Print.
- https://sciencenotes.org/elementary-reaction-definition-and-examples-chemistry/
- https://iopscience.iop.org/book/mono/978-1-6817-4664-7/chapter/bk978-1-6817-4664-7ch1
- https://pressbooks.bccampus.ca/chbe220/chapter/reaction-mechanisms-elementary-reactions/
- Wayne, R.P. (2002). “Termolecular Addition Reactions.” Encyclopedia of Atmospheric Sciences. Elsevier Science Ltd. ISBN:978-0-12-227090-1.

