These are the compounds in which the central atom lacks eight electrons in its valence shell or possesses eight electrons but can expand the valency due to the presence of valence d-orbital. In short, electron-deficient compounds lack the electrons required to complete the octet of the central atom. The most common electron-deficient compounds are the compounds formed by the boron family i.e., BH3, AlH3, etc., . E.g., Diborane
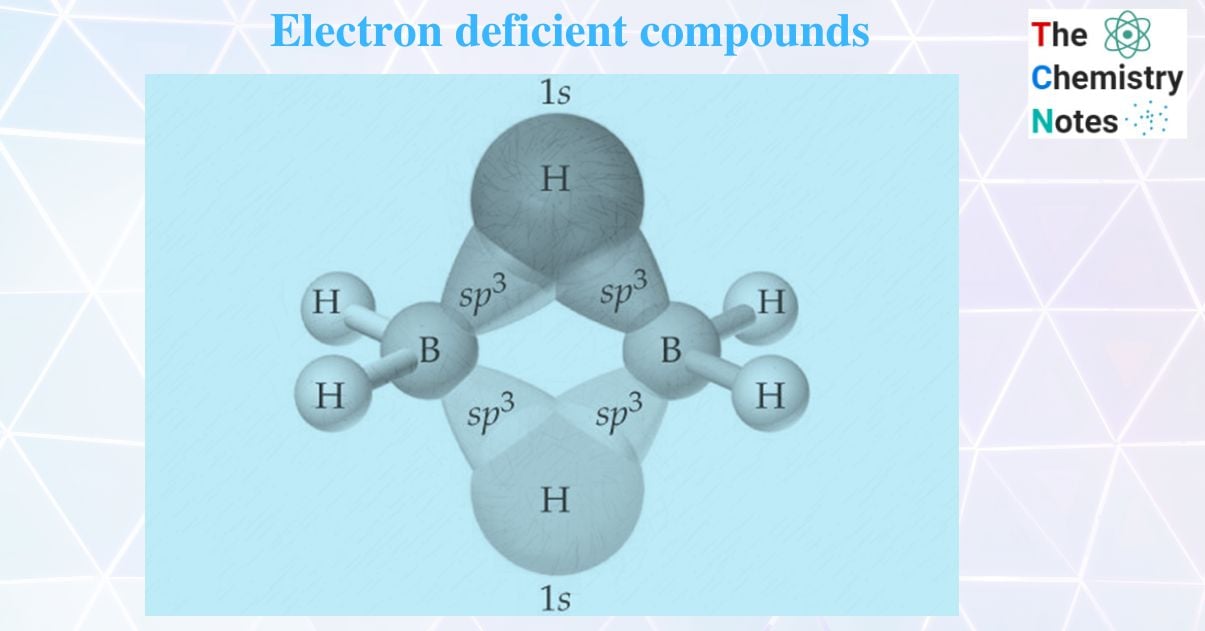
Diborane (B2H6), an electron-deficient compound
There are not enough valence electrons in a diborane molecule to form the expected number of normal covalent bonds between all atoms. The boron atom can form a bond with three hydrogen atoms because it has three electrons in its outermost shell. As a result, each B- atom in diborane can link to three H- atoms. There is no electron to form a bond between two boron atoms. Hence, it is clear that B2H6 contains 12 valence electrons and its 12 valence electrons are insufficient for an ethane-like H3B – BH3 structure which has 7 bonds and is required to have 14 electrons. Hence B2H6 is electron deficient.
Pi back bonding
Π back bonding is a phenomenon in which electrons move from one atom’s atomic orbital to the Π * antibonding orbital of another atom or ligand. It is also known as Π back donation. Metal electrons are used to bond to the ligand, thereby relieving the metal of excess negative charge.
This type of bonding occurs when one atom in a compound has a single pair of electrons and another has an empty orbital next to each other.
Pi – Pi back bonding in BF3
BF3 is electron deficient compound. In BF3, Boron being an atom very small can get effective Π- overlap and attain octet by Π- bonding with any one of the three halogen atoms. This back bonding occurs between unhybridized empty 2Pz orbital on the B-atom, and P- orbital of any one of three halogen atoms.
AlCl3 is also deficient, the size of Al is large so it can not get effective Π- overlap with halogen, and therefore PΠ- P Π bonding. As a result, they dimerize to compensate for the electron deficiency.
3C- 2e Bond
An electron-deficient chemical bond involves three atoms sharing two electrons. Three atomic orbitals combine to form three molecular orbitals, i.e., one bonding, one nonbonding, and one antibonding.
Many common bonds of this type have the bonding orbital shifted toward two of three atoms rather than being spread evenly. E.g., B2H6
Formation of 3C- 2e bond in diborane
Electron diffraction and Ir study shows that B2H6 has hydrogen – bridged structure as:
There are insufficient electrons, so each boron atom bonded to the two terminal hydrogen atoms (Ht) and the other two with a common hydrogen bridge (Hb)and forms a banana bond called the 3c -2e bond.
Valence shell configuration of the Boron atom,
Ground state: 1S2, 2S2, 2PX1, 2PY0, 2PZ0,
Excited state: 1S2, 2S1, 2PX1, 2PY1, 2PZ0,
The four atomic orbitals hybridize together to give four SP3 hybrid orbitals in which one is empty and the remaining three are singly filled. Hence each boron atom uses SP3 hybrid orbitals.
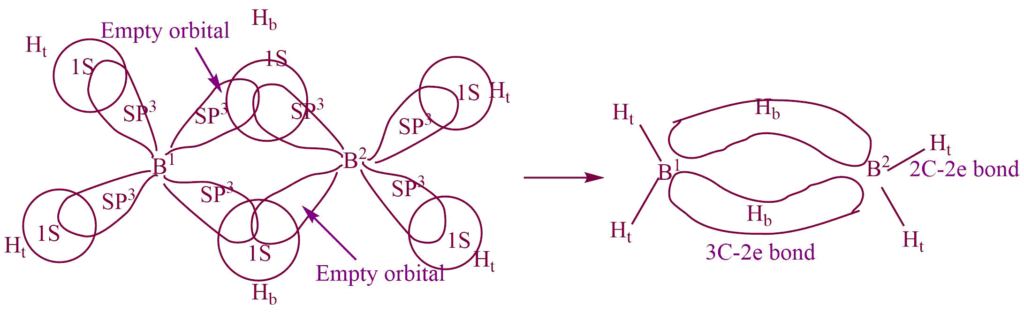
One singly filled SP3 hybrid orbital on the B1 atom and one empty SP3 hybrid orbital on the B2 atom and a singly filled 1S orbital on one Hb atom overlap together and form one bridging 3c -2e bond.
Similarly, one empty SP3 hybrid orbital on the B1 atom and one singly filled SP3 hybrid orbital on the B2 atom, and a singly filled 1S orbital on another Hb atom overlap together and form another bridging 3c -2e bond. The remaining two singly filled SP3 hybrid orbital of each B- atom overlap with the singly filled SP3 1S orbital of the Ht atom to form a normal 2c- 2e bond.
Suggested video
References
- Cotton F. A. (1999). Advanced inorganic chemistry (6th ed.). Wiley. Retrieved November 12 2022 from http://catdir.loc.gov/catdir/bios/wiley042/98008020.html.
- Haaland, Arne, ‘Electron deficient molecules: three-center, two-electron bonds’, Molecules and Models: The molecular structures of main group element compounds (Oxford, 2008; online edn, Oxford Academic, 1 May 2008), https://doi.org/10.1093/acprof:oso/9780199235353.003.0012, accessed 12 Nov. 2022.
- https://facultyfp.salisbury.edu/dfrieck/htdocs/212/elements/boron/b2h6.htm
- https://healy.create.stedwards.edu/Chemistry/MMedia/AdvOrganicProject/ParkinJChemEd.pdf
- https://byjus.com/jee/back-bonding/
- https://www.chemeurope.com/en/encyclopedia/%CE%A0_backbonding.html

