The arrangement of an atom’s or molecule’s (or other physical structure’s) electrons in their atomic or molecular orbitals is known as the electron configuration. Electron configuration is also known as electronic structure or electronic configuration.
What is Electron Configuration?
An element’s electron configuration describes the distribution of electrons in its atomic orbitals. Atoms’ electron configurations are represented using a standard designation, which arranges all atomic subshells that contain electrons in a sequence according to the number of electrons each one is able to hold. The electron configuration provides details on the number of electrons in each of an atom’s shells, subshells, and orbitals.
Electron Configuration of an element tells us how electrons are filled inside various orbitals of the atom. The distribution of electrons inside various orbital of atoms is very useful in explaining various properties of the atoms and their combination with other atoms.
The electron configuration of the N (the most common element in the Earth’s atmosphere) is 1s2 2s2 2p4 as it has 7 electrons which are arranged in order as 2 is 1s orbital, 2 in 2s orbital, and 3 in 2p orbital.
Rules for Electron Configuration
The following general guidelines can be used to determine the electron configuration of an atomic species: Pauli-Exclusion Principle, Hund’s Rule, and the Aufbau Principle. Before moving on, it’s critical to realize that each orbital can accommodate two electrons with opposite spins. The number of electrons that could fit into each orbital in a specific subshell is displayed in the table below.
| subshell | total number of possible electrons in each orbital |
| s | 2 |
| p | 6 |
| d | 10 |
| f | 14 |
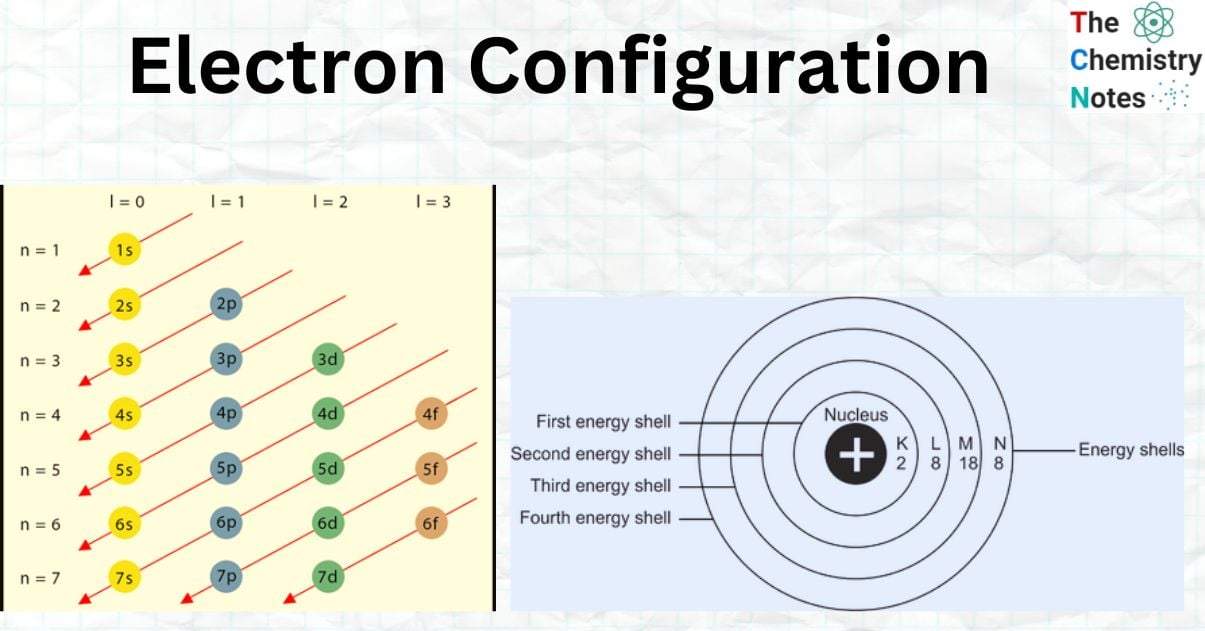
Aufbau Principle
According to the Aufbau Principle, electrons fill the lowest energy levels of an atom first and the highest energy levels last. Electrons may occupy four different sorts of subshell forms and seven different energy levels.
Thus, the Aufbau principle specifies how electrons are positioned in the ground state atomic orbitals of an atom. As a result, electrons are put into atomic orbitals in the order of climbing orbital energy. According to the Aufbau principle, accessible atomic orbitals with the lowest energy levels are filled before those with higher energy levels.
Pauli Exclusion Principle
According to the Pauli Exclusion Principle, an orbit with opposite spins can only hold two electrons. It is based on the idea that if two electrons have the same azimuthal number, they will have opposing spins in their orbit.
Hund’s Rule
According to the rule, each orbit is initially occupied by a single electron before being filled by a second electron. The order of electrons in the sub-shell is determined by this law.
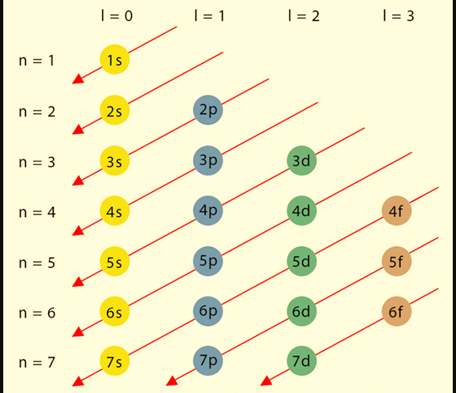
Distribution of Electrons in Shells
Since Electron configuration, also known as electronic configuration, is the arrangement of electrons within the atom in shells, subshells, and orbitals. Let us discuss the components.
Electron Shells
Electron shells are frequently referred to as energy levels. Each shell has its own primary quantum number. As shells move away from the nucleus, their principal quantum number increases and their energy level rises.
Electron sub-shells
Sub-shells are subdivisions of each shell. They also have various energy levels: the s sub-shell has the least energy, followed by p, d, and f. The number of orbitals in each sub-shell varies. The s sub-shell, for example, contains only one orbital, whereas the p and d sub-shells each have three and five, respectively.
Orbitals of electrons
Orbitals are areas of space in which an electron can be detected 95% of the time. Each orbital can only hold two electrons. These electrons must have opposite spins – one with an up spin and the other with a down spin. Orbitals can also vary in shape based on their subshell.
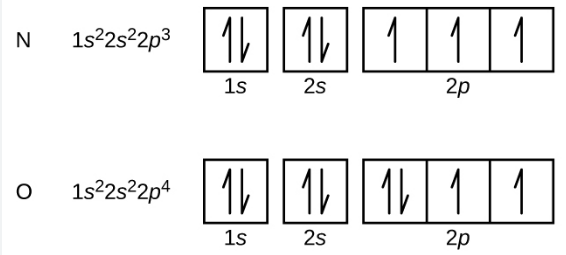
Electron Box Notation
The electron configuration can alternatively be expressed using the electrons in boxes notation. Each box represents an atomic orbital. The boxes are organized in ascending order of increasing energy from lowest to greatest.
To demonstrate the spin of the electrons, opposite arrows are used to symbolize them.
As an example, the box notation for Nitrogen and Oxygen is given below:
References
- Atkins, P. W., & De Paula, J. (2006). Physical Chemistry for the Life Sciences. New York, NY: W. H. Freeman and Company.
- Petrucci, R. H., Harwood, W. S., & Herring, F. G. (2002). General Chemistry: Principles and Modern Applications. Upper Saddle River, NJ: Prentice-Hall, Inc.
- Shagoury, Richard. Chemistry 1A Lecture Book. 4th Ed. Custom Publishing. 2006. Print
- https://www.geeksforgeeks.org/electron-configuration/
- Rayner-Canham, Geoff; Overton, Tina (2014). Descriptive Inorganic Chemistry (6 ed.). Macmillan Education. ISBN 978-1-319-15411-0.
- https://alevelchemistry.co.uk/notes/electron-configurations/
- https://www.savemyexams.co.uk/as/chemistry/cie/22/revision-notes/1-physical-chemistry/1-1-atomic-structure/1-1-8-electron-configuration/
