The electromagnetic spectrum can be defined as the set of frequencies from electromagnetic radiation and the accompanying wavelengths and photon energies. Typically, electromagnetic waves move at speeds comparable to those of light in a vacuum. However, they operate at several wavelengths, frequencies, and photon intensities.
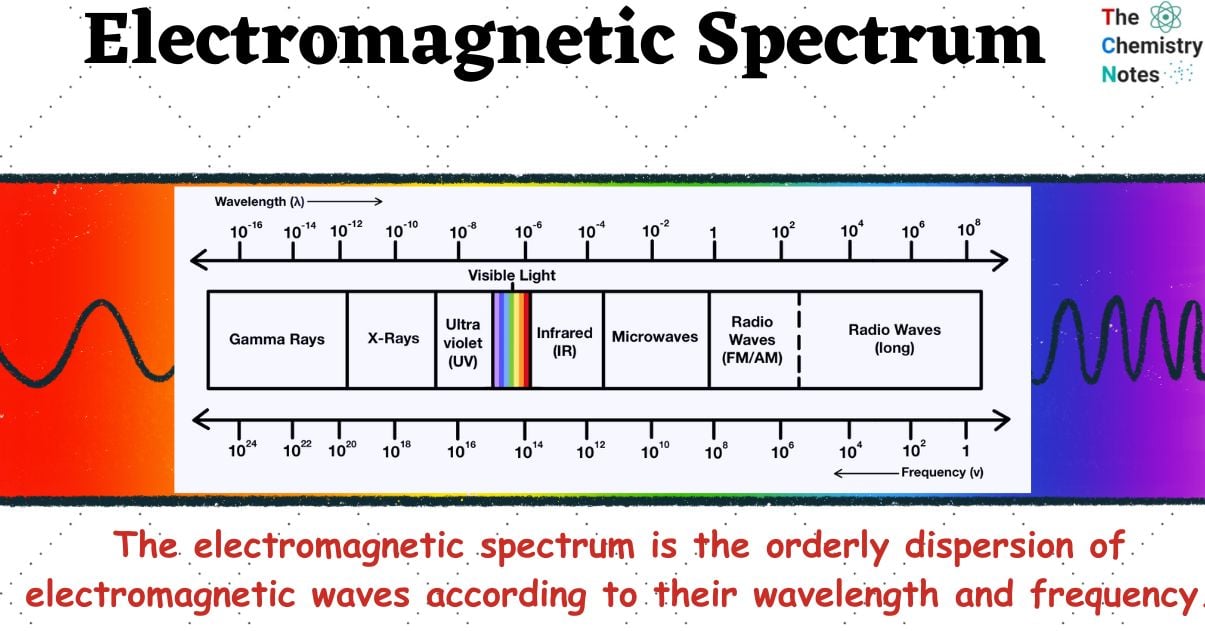
The electromagnetic spectrum is a range of all electromagnetic radiation that includes numerous subranges, sometimes known as portions. These can also be further categorized as ultraviolet radiation, visible light, and infrared light. Visible light waves are the only waves that are visible to human eyes.
What is Electromagnetic Spectrum?
The electromagnetic spectrum is a collection of wavelengths, photon energies, and frequencies that range from below 1 Hz to above 1025 Hz. This corresponds to wavelengths in the electromagnetic wave spectrum that range from a few kilometers to a minuscule fraction of an atomic nucleus.
In other words, the electromagnetic spectrum is the orderly dispersion of electromagnetic waves according to their wavelength and frequency.
Range Of Electromagnetic Spectrum
- The electromagnetic spectrum is the range of all possible frequencies of electromagnetic radiation. The characteristic distribution of electromagnetic radiation that an object emits or absorbs has a different meaning than the term “electromagnetic spectrum” generally used.
- The significance of the electromagnetic spectrum is that it can be used to classify electromagnetic waves and arrange them according to their different frequencies or wavelengths.
The types of radiation and their frequency and wavelength ranges are as follows:
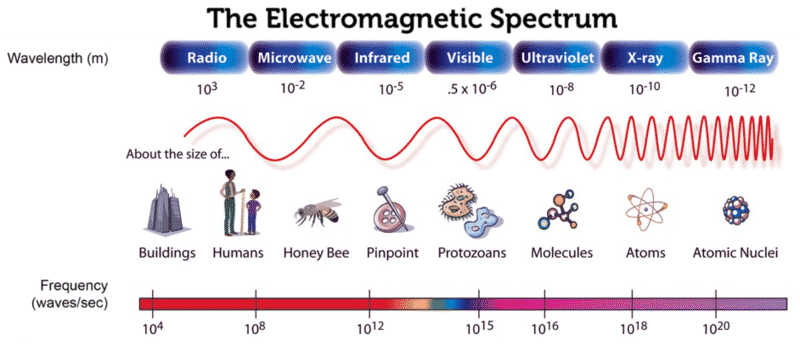
| Type of radiation | Frequency Range in Hz | Wavelength Range |
|---|---|---|
| Gamma-rays | 1020 – 1024 | < 10-12 m |
| X-rays | 1017 – 1020 | 1 nm – 1 pm |
| Ultraviolet | 1015 – 1017 | 400 nm – 1 nm |
| Visible | 4 x 1014 – 7.5 x 1014 | 750 nm – 400 nm |
| Infrared | 1013 – 1014 | 25 μm – 2.5 μm |
| Microwaves | 3 x 1011 – 1013 | 1 mm – 25 μm |
| Radio waves | < 3 x 1011 | > 1 mm |
Waves In The Electromagnetic Spectrum
Let us discuss the use of electromagnetic waves that are around us.
Radio-Waves
- Radio waves have frequencies that span from 500 kHz to around 1000 MHz (< 3 x 1011).
- They are the result of charges moving through conducting wires at a faster pace.
- Cellular phones utilize radio waves to transmit voice communication within the ultrahigh-frequency (UHF) band.
- Radio receivers detect and capture radio waves that are transmitted by radio stations. The vast majority of radio waves is allocated for the purposes of television and mobile communication.
- Stars and gas in the solar system can also emit radio waves.
Microwaves
- Microwaves are essentially short-wavelength radio waves.
- They have a frequency range of between (3 x 1011 – 1013 ) Hz.
- These radiations are used for aircraft navigation radar systems, speed testing (via speed guns), and, most significantly, in the kitchen appliance, the microwave.
- Astronomers also use it to determine and comprehend the structure of nearby galaxies and stars.
- Klystrons, magnetrons, and Gunn diodes are distinct vacuum tubes that generate electromagnetic radiation.
Infrared
- The frequency range of infrared waves is (1013–1014 )Hz.
- These are also known as “heat waves.” Usually, hot bodies and molecules produce them.
- These waves are used in Earth satellites, night vision goggles, remote switches for household appliances, etc.
- Infrared waves help maintain the earth’s warmth and average temperature. Radiations are trapped in the earth’s atmosphere by greenhouse gases, including carbon dioxide and water vapor.
- These radiations are utilized by night vision equipment. These devices are capable of detecting and recording infrared radiation emitted by objects in low-light conditions. Thus,in space, infrared light helps to map interstellar dust.
Visible Rays
- The range of frequencies for visible light is (4 x 1014 – 7.5 x 1014) Hz. The wavelength range of visible rays is 400nm–700nm.
- Since human eyes can detect visible light, these rays are the most well-known.
- Visible rays are those that luminaries like lights, stars, and other objects emit or reflect.
- Various devices that produce light within the visible range of the electromagnetic spectrum encompass but are not limited to bulbs, lamps, candles, LEDs, and tube lights.
Ultraviolet Rays
- The frequency range of ultraviolet rays is (1015 – 1017 )Hz. Ultraviolet radiation is characterized by a range of wavelengths spanning from 400 nanometers to 1 nanometer.
- Hot materials in space also emit UV radiation, including the sun, the major UV ray emitter. When exposed to these rays for extended periods, humans can suffer harm. The ozone layer absorbs these harmful rays, thus protecting us. Water purifiers sometimes use UV lamps to kill the germs in the water.
- UV radiation can have medical applications by being focused into narrow beams for high-precision surgeries such as laser-assisted in situ keratomileusis (LASIK).
- Ultraviolet lamps are employed in water purification systems to eradicate any microorganisms that could potentially exist within the water.
- Welders utilize specialized goggles to safeguard their eyes while working with UV welding arcs.
X-Rays
- X-rays have a frequency range of (1017–1020) Hz.
- They are most commonly used for diagnostic purposes in the medical world. X-ray imaging is a medical diagnostic modality that can be efficacious in the management of certain malignancies. In order to identify the origin of an issue, a medical practitioner employs an X-ray imaging device to examine our skeletal structure or dentition.
- Excessive exposure to x-rays can result in detrimental effects or fatality to the healthy tissues of the organism. Consequently, it is imperative to exercise utmost care while handling x-rays.
- X-rays can be generated by subjecting a metal target to high-energy electrons.
- At the airport security checkpoint, personnel employ the method to inspect the baggage of travelers.
- There are hot gases in the universe that also emit X-rays. X-rays are emitted by ionized gases in the universe that have been heated to high temperatures.
Gamma-Rays
- Gamma rays occur between (1020 and 1024 )Hz.
- These rays are situated in the higher frequency region of the electromagnetic spectrum. Gamma rays are characterized by their wavelength, which typically falls within the range of 10–12m to 10–14m.
- These are high-frequency radiations that nuclear processes involving radioactive nuclei also produce.
- It can be used in a variety of medical settings. In medicine, they are also employed to eradicate cancerous cells.
Significance of The Electromagnetic Spectrum
- Depending on their origin, interactions with matter, and practical uses, the electromagnetic waves in these various bands exhibit different characteristics.
- Maxwell’s equations predicted that there would be an infinite variety of electromagnetic wave frequencies, all moving at the speed of light. This is the first indication of the existence of the entire electromagnetic spectrum.
- However, the electromagnetic spectrums’ strength lies in their ability to categorize electromagnetic waves and arrange them according to their frequencies or wavelengths.
Applications of Electromagnetic Spectrum
- Hertz is credited with the initial discovery of radio waves and microwaves. The emergence of wireless television and radio, along with mobile communication, can be attributed to the propagation of electromagnetic waves.
- The segment of the electromagnetic spectrum that is perceptible to the human eye, namely visible light, is the fundamental basis for all visual aids utilized in everyday activities. The segment of the electromagnetic spectrum that facilitates visual perception of objects, encompassing the full range of colors, is commonly referred to as visible light.
- Ultraviolet radiation has been found to be a valuable tool in facilitating the ionisation of atoms, thereby playing a crucial role in the initiation of a multitude of chemical reactions.
- X-rays as diagnostic tools are employed to detect anomalies and irregularities in the skeletal system, including bones and teeth.
- The Global Positioning System (GPS) employs radio frequency signals transmitted by orbiting satellites to ascertain the geographical coordinates of terrestrial devices.
- Remote sensing employs electromagnetic waves to acquire data about the Earth and its surroundings from a remote location, as exemplified by the utilization of weather satellites.
Properties of Light Waves
- The characteristics of electromagnetic radiation that are visible to the human eye are commonly referred to as light. These properties include wavelength, frequency, amplitude, polarization, and speed. Understanding the properties of light is essential in various fields such as physics, chemistry, and biology.
- In the discourse of optics, the customary terminology employed to describe light pertains to its wavelength and frequency. The utilization of these two terminologies can facilitate the depiction of the behavior of light.
- The concept of light is intriguing in the realm of quantum mechanics due to its dual nature as both a particle, namely the photon, and a wave. The phenomenon being referred to is commonly known as particle-wave duality.
- When conceptualizing a wave, it is advantageous to envision it as a sinusoidal waveform, exhibiting periodic oscillations.
Basic Properties of Waves
The three physical properties of electromagnetic waves—frequency (f), wavelength, or photon energy—typically characterize them.
Amplitude:
- The term “amplitude” pertains to the vertical distance between the highest and lowest points of a wave in relation to its midline.
- The intensity or brightness of light is determined by the amplitude of the corresponding wave.
- The luminosity of light is directly proportional to its amplitude.
Wavelength:
- The term “wavelength” (λ) denotes the distance between two consecutive points on a wave that are in phase, corresponding to one complete cycle of the wave.
- The distance can be characterized as either the peak-to-peak or zero-to-zero distance.
- The standard unit of measurement for it is nanometers (nm); however, alternative units of measurement such as meters or micrometers can also be utilized.
- The color of light is determined by the wavelength of the wave.
Frequency:
- The physical quantity denoted by the symbol “frequency (v)” refers to the count of wave oscillations that traverse a stationary point within a specified duration, and its unit of measurement is expressed in cycles per second (s^-1) or Hertz (Hz).
- The unit of frequency known as hertz is precisely defined as the occurrence of one complete cycle within a time frame of one second.
- The relationship between the frequency of a wave and its speed is one of direct proportionality.
- In essence, there exists a direct relationship between the speed of a wave and its frequency, whereby an increase in wave speed results in a corresponding increase in frequency.
Formulas for Electromagnetic Waves
The light’s energy can be calculated by the equation:
E = h.c / λ
where,
- E is the light’s energy
- h is the Planck’s constant
- c is the light’s velocity
- λ is the wavelength
The other forms of equation are:
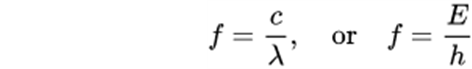
Frequently Asked Questions (FAQ)
Which part of the electromagnetic spectrum has the longest wavelengths?
Radio waves are the type of electromagnetic radiation with the longest wavelengths. The wavelengths of radio waves range from around 1 millimeter to over 100 kilometers. They are frequently employed in radio and television transmissions, cellular phone networks, and satellite communications, among other forms of broadcasting and communication.
Where is visible light located on the electromagnetic spectrum?
Between the infrared (IR) and ultraviolet (UV) portions of the electromagnetic spectrum lies visible light. Its frequencies range from 4 × 1014 to 8 × 1014 hertz (Hz), or cycles per second, and its wavelengths range from 740 nanometers (nm) or 2.9 × 10−5 inches, to 380 nm (1.5 × 10−5 inches).
Video on Electromagnetic Spectrum
References
- Theodore Lawrence Brown, Eugene, H., Bursten, B. E., Murphy, C. J., Woodward, P. M., Stoltzfus, M. W., & Lufaso, M. W. (2018). Chemistry : the central science (14th ed.). Pearson.
- https://byjus.com/jee/electromagnetic-spectrum-and-electromagnetic-waves/
- Malone, L. J., & Dolter, T. O. (2010). Basic concepts of chemistry. Wiley.
- https://hubblesite.org/contents/articles/the-electromagnetic-spectrum
- https://economictimes.indiatimes.com/definition/electromagnetic-waves
- https://www.ck12.org/physics/electromagnetic-spectrum-in-physics/lesson/Electromagnetic-Spectrum/
- https://www.geeksforgeeks.org/electromagnetic-spectrum/
- https://tuitionphysics.com/oct-2020/electromagnetic-waves-definition-applications-concepts/
- https://library.fiveable.me/ap-chem/unit-3/spectroscopy-electromagnetic-spectrum/study-guide/


Insightful content. Kudos 👌👍