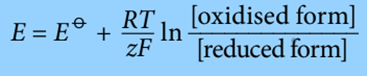The electromotive force of a cell made up of two electrodes in electrochemistry is known as the electrode potential. Conventionally, the standard hydrogen electrode is used as the reference electrode (SHE). It is stated that it has a potential of 0 volts. It can also be described as the potential difference between the salt solution and the charged metallic rods.
Electrode Potential
In electrochemistry, Electrode potential is the electromotive force of a galvanic cell constructed from a reference electrode that is considered to be standard and a test electrode.
The potential difference, measured in volts (v), is determined by the materials used to make the electrodes. The sum of the potentials created by the reactions at the two electrodes is the total potential for any electric cell:
EMF cell = EMF oxidation + EMF reduction
Electromotive force, or EMF, is another name for electrical potential.
Chemists have connected each electrode in a cell with a standard hydrogen electrode, which is hydrogen gas bubbling over a platinum wire submerged in 1 M H+ (aqueous) at 1 atmosphere, to measure the voltages of a wide range of electrodes. The potential of the other electrode can be determined by measuring the EMF of the entire cell, which is given an arbitrary potential of 0 volts for this standard electrode.
Types of Electrode Potential
There are two different types of electrode potential, depending on whether a metal electrode will lose or gain electrons.
Oxidation potential
An electrode functions as an anode when it is negatively charged in relation to a solution. The oxidation takes place.
M → Mn+ + ne–
Reduction potential
An electrode functions as a cathode when it has a positive charge relative to the solution. There is a reduction.
Mn+ + ne– → M
Standard electrode potential
The standard electrode potential, typically represented by E°, if in the half cell, a metal rod (M) is suspended in a solution of one molar concentration, and the temperature is at 298 K.
The potential difference in volts created in a cell with two electrodes, the pure metal in contact with a molar solution of one of its ions and the typical hydrogen electrode, is known as the metal’s standard electrode potential (NHE).
Reference half-cells or a reference electrode
The absolute value of single electrode potential cannot be directly measured. Experimental measurements of the only difference in potential between two electrodes are possible. The electrode must therefore be connected to another electrode whose potential is known. This electrode is also known as a reference half-cell or reference electrode. Various types of half-cells have been used to create complete cells with spontaneous forward reactions.
Standard Electrode Potential
A measurement of the potential for equilibrium is the standard electrode potential. The potential of the electrode is the difference in potential between the electrode and the electrolyte. The electrode potential is referred to as the standard electrode potential when unity represents the concentrations of all the species involved in a semi-cell.
In an electrochemical cell, the standard electrode potential occurs at, for example, 298 K, 1 atm of pressure, and 1 M of concentration. The typical electrode potential of a cell is denoted by the symbol “E°cell“
A reaction’s equilibrium position could be impacted by alterations in reagent concentration, temperature, and gas pressure, etc. The voltage of an electrochemical
cell will also depend on these factors, so we should use standard conditions
when comparing electrode potentials.
- concentration of ions at 1.00 mol dm–3
- temperature of 25°C (298K)
- any gases should be at a pressure of 1 atmosphere (101 k Pa)
- the value of the electrode potential of the half-cell is measured relative to the standard hydrogen electrode.
Overall the electrode potential we observe in these situations is the standard electrode potential
Electrode potential and redox reactions
Values of the electrode potential give us an indication as to how simple it is to reduce a substance.
Note that:
The reduction reaction is typically described in terms of the electrode potential. In the resulting half-equation, the electrons are visible on the left side.
For example: Al3+ (aq) + 3e– ⇌ Al (s)
The more positive (or less negative) the electrode potential, thus the easier it is to reduce the ions on the left. So, the metal on the right is relatively unreactive and is a relatively poor reducing agent.
For example: Ag+ (aq) + e– ⇌ Ag (s) voltage = +0.80V
The more negative (or less positive) the electrode potential, hence the more difficult it is to reduce the ions on the left. So, the metal on the right is relatively reactive and is a relatively good reducing agent.
For example: Zn2+ (aq) + 2e– ⇌ Zn (s) voltage = –0.76V
Factors Affecting Electrode Potential
- The position of the redox equilibrium and, consequently, the electrode potential of the half-cell, will be changed by changes in temperature, pressure, or concentration.
- The electrode potential increases if the position of equilibrium is shifted in favor of the forward reaction (the reduction).
- The electrode potential shifts more negatively if, on the other hand, the position of equilibrium is shifted in favor of the backward reaction (the oxidation).
Hence, Nernst equation can be used to forecast a half cell’s electrode potential in unusual circumstances:
Where:
E = electrode potential under non-standard conditions
Eꝋ= standard electrode potential
R = gas constant (8.314 JK-¹mol-¹)
T = Temperature in Kelvin
Z = number of electrons transferred
F = Faraday Constant in C mol-¹
Ln = Natural logarithm
[oxidised] = concentration in mol dm–³ of the oxidised form in the half equation.
Applications of Standard Electrode Potentials
Some of the uses for common electrode potentials are as follows:
- It serves as a tool for comparing the relative potencies of different oxidants and reductants.
- It is used to determine how to calculate the average cell potential.
- Moreover, it is used to predict potential outcomes.
- This is also used for the prediction of the equilibrium of the reaction.
The Importance of Standard Electrode Potential
- Redox reactions, which are composed of two half-reactions, are the foundation of all electrochemical cells.
- At the anode, there is an oxidation half-reaction that results in an electron loss.
- At the cathode, a reduction reaction occurs that results in an electron gain. The anode to the cathode is where the electrons move as a result.
- The difference in the individual potentials of each electrode causes an electric potential to develop between the anode and the cathode (which are dipped in their respective electrolytes).
- With the aid of a voltmeter, the cell potential of an electrochemical cell can be determined but a half-individual cell’s potential, however, cannot be precisely measured on its own.
- It’s also critical to remember that this potential can therefore change in response to modifications in pressure, temperature, or concentration.
- The requirement for E° arises in order to obtain the individual reduction potential of a half-cell.
- With the aid of a reference electrode known as the standard hydrogen electrode, it is measured (abbreviated to SHE). SHE has an electrode potential of 0 volts.
- By connecting an electrode to the SHE and measuring the cell potential of the resulting galvanic cell, the standard electrode potential of the electrode can be determined.
- The opposite of an electrode’s reduction potential is its oxidation potential. As a result, an electrode’s standard reduction potential can be used to describe its E°.
- High standard reduction potentials are exhibited by good oxidizing agents whereas low standard reduction potentials are exhibited by good reducing agents.
Grab overall information on electrochemistry https://youtu.be/IV4IUsholjg
References
- https://byjus.com/chemistry/standard-electrode potential/https://byjus.com/chemistry/standard-electrode-potential/
- C.A. Hamel, “The Encyclopedia of Electrochemistry”, Reinhold Publishing Corporation, New York-Chapman & Hall Ltd., London, 1964,
- https://www.studysmarter.co.uk/explanations/chemistry/physical-chemistry/electrode-potential/
- https://en.wikipedia.org/wiki/Electrode_potential