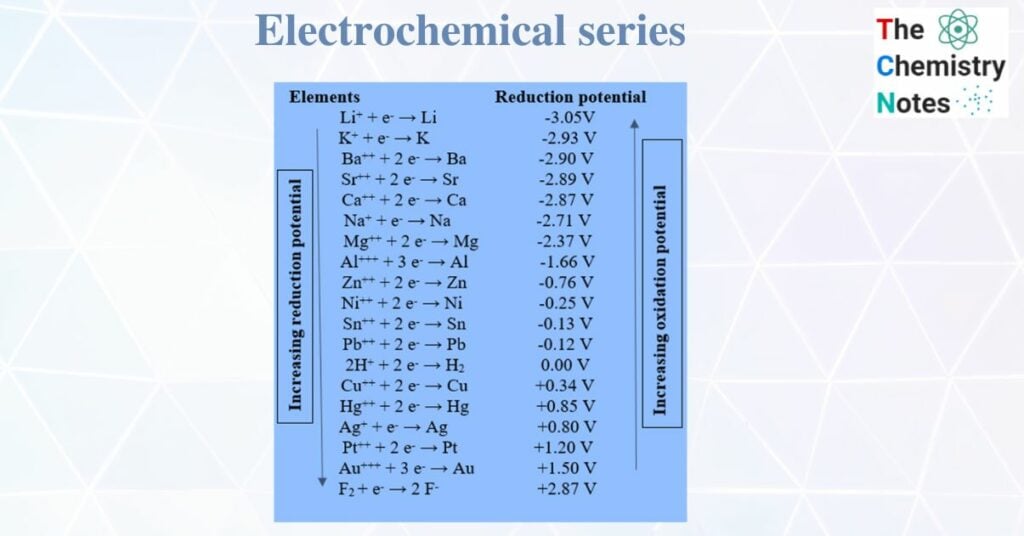
The series of elements or ions in the increasing order of reduction potential or decreasing order of oxidation potential is known as the electrochemical series. From the electrochemical series, we can predict the feasibility of the reaction, the emf of the cell, and the formation of hydrogen gas from dilute mineral acid by metal, etc.
Interesting Science Videos
Application of electrochemical series
1. To predict whether the metal can or can not displace hydrogen gas from dilute mineral acid
The metal lying above the hydrogen in the electrochemical series can displace hydrogen gas from dilute mineral acid i.e.,
Zn + dil. H2SO4 →ZnSO4 + H2
Cu + dil. H2SO4 →No reaction
2. To calculate the Emf of cell
Emf of a cell can be calculated by the following formula.
EoCell = Eocathode – Eoanode
For example, the standard reduction potential of Eo Ag+/ Ag = 0.80 V and Eo Ni++/ Ni = 0.25 V. Find the cell potential at standard conditions.
EoCell = Eocathode – Eoanode
= 0.80 + 0.25
= 1.05
3. To predict anode and cathode
The metal having a lower value of reduction potential acts as an anode whereas the metal with a higher value of reduction potential acts as the cathode. Among Zinc and copper, copper acts as a cathode whereas Zinc acts as an anode as copper has a higher reduction potential.
3. To predict oxidizing and reducing agent
The metals or ions having lower values of reduction potential act as reducing agents whereas the metals or ions having higher values of reduction potential act as oxidizing agents. From the above electrochemical series, Lithium is the best reducing agent whereas Fluorine is the most powerful oxidizing agent.
4. To predict whether a metal can or can not displace another metal in a salt solution
The metal which is less electropositive than the metal present in the salt solution can displace metal from its salt.
Zn + CuSO4 → ZnSO4 + Cu
Cu + ZnSO4 → No reaction
Thus zinc sulfate can be stored in a copper vessel but copper sulfate can not be stored in a zinc vessel as it forms an electrochemical cell.
4. To predict the feasibility of the reaction
To predict the feasibility of the reaction, the emf of a cell can be calculated. If the value of emf is positive then the reaction is feasible. If the emf is negative then the reaction will not occur.
e.g.,
Ni++ + Cu → Cu++ + Ni
Given, Eo Ni++/ Ni = -0.25 V
Eo Cu++/ Cu= -0.34 V
Here cu acts as an anode and Ni acts cathode.
EoCell = EoCathode – Eoanode
= -0.25 – 0.34
= -0.59 V
Since the value of standard electrode potential is negative, the reaction is not feasible.
Suggested video
References
- Bahl A. Bahl B. S. & Tuli G. D. (2008). Essentials of physical chemistry. S. Chand.
- https://byjus.com/jee/electrochemical-series/
- https://thefactfactor.com/facts/pure_science/chemistry/physical-chemistry/electrochemical-series/5877/
- https://www.elearn.gov.pk/onlinepdf/chemistry/11chemistry/pdfEng/chapter10.pdf
- https://www.sji.edu.sg/qql/slot/u560/News%20and%20Events/News%20Highlights/2019/Talbot%207352%20SSR_reactivity_series_activity_series_electrochemical_series.pdf

