Ecotoxicology combines elements of ecology, toxicology, physiology, analytical chemistry, molecular biology, and mathematics. Ecotoxicology studies how pollutants, such as pesticides, affect people, populations, the environment, and ecosystems. Ecosystems are made up of groups of living beings and the habitats they occupy. Ponds, rivers, deserts, grasslands, and forests are examples of ecosystems, and pesticides can have an impact on any of them. Ecotoxicologists also investigate what happens to the pesticides themselves, including where they end up in the environment, how long they persist, and how they eventually degrade.
Interesting Science Videos
What is Ecotoxicological?
The study of the negative effects of chemicals on ecosystems is known as ecotoxicology. This means that ecotoxicology is concerned with the biological impacts of toxic substances that contaminate or have contaminated the environment. These biological consequences can range from a molecular effect in a species to effects on the entire biosphere.
- Ecotoxicology, a part of environmental chemistry is the study of pollutants in the biosphere and their effects on biosphere constituents. Its overall purpose is to describe and anticipate effect or exposure events at various levels of biological organization.
- The consequences relevant to nonhuman targets range from biomolecular to global. More cause-effect models relevant to these higher levels of biological organization are being added to the conventional set of toxicology models used by pioneering ecotoxicologists as the need to predict major effects on populations, communities, ecosystems, and other higher level entities becomes more apparent.
- Contaminant chemical form, phase association, and biosphere mobility are also important concerns in ecotoxicology because they influence exposure, bioavailability, and realized dose.
Toxic Elements and Compounds
Ozone, white phosphorous, elemental halogens (fluorine and bromine), and heavy metals (cadmium, lead, arsenic, and mercury) are all part of toxic elements. Lead is one of the most common toxins on the planet. Vehicle exhaust, home paint, ceramic glazing, batteries, cosmetics, lead mining, smelting, and coal combustion all contribute to its presence in the environment. Lead inhalation reduces intelligence and causes heart problems in humans.
Toxic Inorganic compounds
Toxic inorganic chemicals include hydrogen cyanide, carbon monoxide, nitrogen oxides (NO and NO2), hydrogen halides, and others.
Silica can be found in a number of rocks. When people are exposed to silica dust, it produces an occupational disease known as silicosis. Asbestos is a category of fibrous silicates that are extensively employed as fire retardants, home flooring, and walls. Inhaling asbestos particles causes asbestosis. It is a degenerative lung condition that causes lung cancer.
Toxic Organic Compounds
Organic solvents that are carcinogenic include benzene, carbon tetrachloride, and trichloroethylene. Organic solvents known to be carcinogenic to humans include 2-ethoxyethanol, 2-methoxyethanol, and methyl chloride. Among the organic solvents known to be neurotoxins include n-hexane, tetrachloroethylene, and toluene. Formaldehyde causes hypersensitivity and lung cancer. Parathion and Malathion cause skin and respiratory problems.
Radionuclides
These are unstable isotopes of elements that continually decay and produce one or more types of radiation. These are either naturally occurring or generated in nuclear power plants, nuclear weapons use, research, and medical uses. It contains Uranium (U), Krypton (Kr), Plutonium (Pu), Thorium (Th), and many more elements.
Routes of Exposure of Chemicals
Chemicals must enter your body to cause health problems. Once in your body, toxins can enter your bloodstream and reach internal “target” organs such the lungs, liver, kidneys, or nervous system. There are three primary routes of exposure, or methods for a chemical to enter your body.
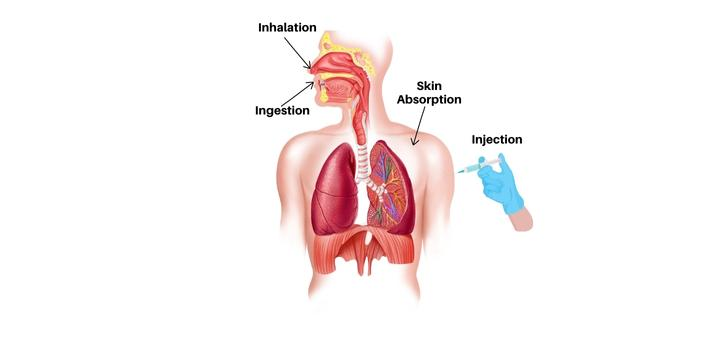
The first pathway is inhalation, which occurs when you breathe in chemical fumes, mists, or dusts in the air. Inhaled chemicals can quickly move from the air sacs into capillaries and reach the bloodstream because the air sacs in the lungs are designed for gas exchange and consist of only a single layer of thin cells. Toxins inhaled can also cause local harm to the tongue, airways, and lungs.
The second pathway is ingestion, which occurs when chemicals are spilled or settle on food, beverages, cigarettes, beards, or hands. Toxins can then cause local damage to the digestive tract before being absorbed into the bloodstream, most commonly through the intestines.
The third pathway is Skin or eye contact. When chemicals come into contact with the skin or the eyes, they can cause regional injury or be absorbed into the bloodstream through the skin. Toxins enter the body through the skin more difficultly than by inhalation or ingestion because the skin is made up of multiple cell layers that are fortified with defensive chemicals. Toxins, on the other hand, can easily penetrate through broken skin, and some substances can cause cancer.
- Toxic compounds enter the bodies of living beings through a variety of routes, including water, air, food, accidental exposure, therapeutic medications, and others. harmful chemical solubility governs harmful chemical movement in live beings’ bodies and diverse components of the environment.
- Water soluble substances spread quickly in both aquatic and terrestrial systems, as well as within the bodies of live animals.
- Fat-soluble substances that are not utilised during metabolic processes reach the tissues.
- Some harmful compounds stay in the environment for a longer period of time.
Chlorofluorocarbons, chlorinated hydrocarbons, and insecticides are examples of chemicals. When they are present in the same environment, they have an additive influence. It is referred to as synergistic effects when one chemical that is less harmful when combined with another chemical exhibits toxicity that is several times greater than the solo effects.
Relationship Between Dose and Response
The chemical dosage indicates the extent of a poisonous substance’s unfavorable effect on an organism. To investigate the time-dependent influence of exogenous and endogenous chemicals and physical agents, the dose response relationship is used. Dose is defined as the amount of toxicant exposed to an organism per unit of body weight. A lead dose to a bird, for example, could be 5 mg Pb/kg body weight. Dose is also given as dose per unit time. (For example, 5mg lead/kg body weight/day). The dosage response is a quantitative relationship that recognizes that the action of certain substances is proportional to the concentration of hazardous components.
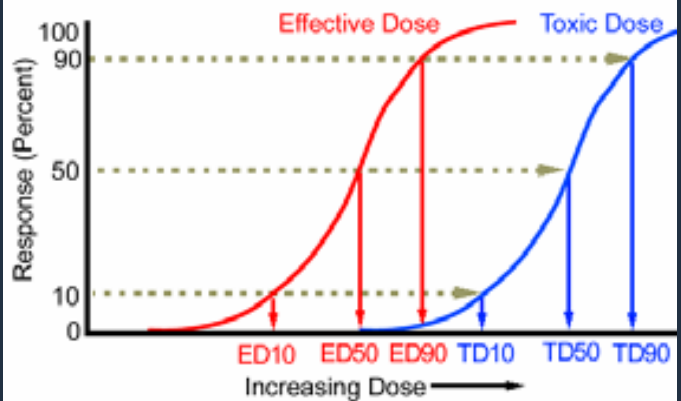
A dosage response curve can be used to demonstrate dose dependency (for example, by administering different doses of a toxicant to a uniform population of test animals and plotting the cumulative percentage of mortality as a function of log of the dose). The dosage response curve is often S-shaped. The dose corresponding to the midway, denoted as LD50, is a statistical estimate of the dose that kills 50% of the population. The dose response curve may differ between different types and strains of organisms, tissues, and cell populations.
Effects of Toxins in Aquatic system
A vast number of pollutants and toxicants are released into rivers, lakes, estuaries, and seas as a result of human activities.
- Heavy metals such as lead, cadmium, and mercury have been shown to accumulate in aquatic life.
- Clams prone to accumulate mercury.
- Pesticides have an impact on crustaceans, fish, and mollusks.
- Oil pollution (oil spill) has a negative impact on echinoderms.
- It is also well recognized that pesticides and heavy metals accumulate in the aquatic system via the food chain.
Effects of Toxins in Human Health
A large number of toxicants or pesticides are ingested, inhaled, or absorbed via the skin by humans. These toxins typically affect one or more organs, such as the lungs, skin, or alimentary canal.
- Asbestos inhalation affects the lungs, causing inflammation in the lung lining and, eventually, lung dysfunction. When skin cells are irritated, asbestos can cause dermatitis.
- Toxins that enter the body are metabolized, transferred, or eliminated. They have negative biochemical effects during the metabolic process and cause poisoning.
- These processes have a kinetic and a dynamic phase. A dangerous chemical may go through absorption, metabolism, temporary storage, distribution, and excretion during the kinetic phase. When a toxicant remains intact during the metabolic process, it becomes an active parent molecule and is ready for subsequent biochemical interactions.
- Some toxins are detoxified and expelled, while others are transformed into non-toxic active metabolites.
Ecological Risk Assessment
Ecotoxicological knowledge is used by ERA to enhance environmental decision making.
- A widely diffused eco toxicant, such as acid rain, or a widely used product, such as a herbicide, may necessitate risk assessment at the landscape or subcontinental scale.
- Eco toxicants needing a worldwide ERA could include greenhouse gases that contribute to global warming, hydrofluorocarbons that deplete the ozone layer, and persistent organic pollutants that accumulate to dangerous levels in polar regions distant from their release points at industrialized latitudes.
- Predictive ERAs, on the other hand, estimate the potential risk associated with a future or planned toxicant exposure.
- Contact between the toxicant and the assessment endpoint is described or predicted by exposure characterization.
- Toxicant sources, transportation modes, contact types, and potential stressors are all needed to be specified.
References
- Newman, M. C.; Unger, M. A. Fundametals of ecotoxicology, 2nd ed.; Lewis Publishers: Boca Raton, FL, 2003; pp 53, 76, 95.
- https://www.biologydiscussion.com/ecotoxicology/ecotoxicology-definition-process-episodes-approach-and-fate/86092
- Bradford, D. F.; Heithmar, E. M.; Tallent-Halsell, N. G.; Momplaisir, G-M.; Rosal, C. G.; Varner, K. E.; Nash, M., S.; Riddick, L. A. Temporal patterns and sources of atmospherically deposited pesticides in alpine lakes of the Sierra Nevada, California, U.S.A. Environ. Sci. Technol. 2010, 44, 4609-4614.
- https://bio.libretexts.org/Bookshelves/Ecology/Environmental_Science_(Ha_and_Schleiger)/04%3A_Humans_and_the_Environment/4.04%3A_Environmental_Health/4.4.04%3A_Environmental_Toxicology
- https://epgp.inflibnet.ac.in/epgpdata/uploads/epgp_content/S000035ZO/P000891/M020628/ET/1519034453M41AppliedEcologyEcotoxicologyQuad1.pdf
- https://accesspharmacy.mhmedical.com/content.aspxbookid=449§ionid=39910799#6487047
