The present study concerns the evolution of the Earth’s atmosphere throughout its geological history. The process by which the current atmosphere came from earlier conditions is complicated, but there is a lot of indirect proof about how the Earth’s atmosphere has changed over time. The geological record of ancient sediments and rocks provides evidence of historical alterations in atmospheric composition, which can be attributed to chemical reactions with the Earth’s crust and, more specifically, to biochemical processes linked to the existence of life.

History of Earth’s Atmosphere
The Earth’s atmosphere has undergone significant changes throughout its history.
- The scientific consensus is that the planet Earth underwent formation approximately 5 billion years ago. During the initial 500 million years, a concentrated atmosphere formed as a result of the release of vapor and gases from the planet’s interior through degassing. The atmospheric composition of approximately 3.5 billion years ago is believed to have comprised carbon dioxide (CO2), carbon monoxide (CO), water (H2O), nitrogen (N2), and hydrogen. The hydrosphere is believed to have originated approximately 4 billion years ago through the process of water vapor condensation. The primary characteristic of the ancient environment was the lack of freely available oxygen.
- Approximately one billion years ago, primitive aquatic organisms, known as blue-green algae, initiated the utilization of solar energy to divide H2O and CO2 molecules, resulting in organic compounds and molecular oxygen (O2).
- Photosynthesis is the process by which solar energy is converted into chemical energy in plants. At elevated altitudes, certain oxygen (O2) molecules underwent photodissociation by absorbing energy from the Sun’s ultraviolet (UV) radiation, resulting in the formation of individual oxygen atoms. These atoms react with the residual oxygen (O2) to generate ozone (O3) molecules, which exhibit a high degree of efficacy in the absorption of ultraviolet radiation. The ozone layer enveloping the Earth serves as a protective barrier, safeguarding the planet against the harmful effects of ultraviolet radiation.
- It is believed that 600 million years ago, the requisite quantity of ozone necessary to protect the Earth from biologically harmful ultraviolet radiation was present. This has had a noteworthy impact on the development of life on our planet and is a crucial factor in enabling the current state of life to persist.
Evolution of Earth’s Atmosphere
First Atmosphere
The initial atmospheric conditions of the Earth are referred to as the First atmosphere. The age of this atmosphere is estimated to be approximately 4.57 billion years.
- During the early stages of Earth’s development, the initial atmosphere consisted predominantly of hydrogen and helium (He).
- The aforementioned entity had a relatively brief existence and can be characterized as having an advanced age.
- This layer was dissipated by the heat emanating from the molten crust and the solar wind.
- The presence of a stable atmosphere on a planet cannot be sustained by hydrogen and helium unless the planet exhibits a considerable mass.
- These elements exhibit a higher probability of achieving escape velocity as a result of stochastic thermal fluctuations. This phenomenon partly explains the scarcity of hydrogen and helium in the present-day atmosphere of the Earth.
Secondary Atmosphere
The secondary atmosphere of the Earth is evidenced by the presence of sedimentary deposits associated with water dating back to approximately 3.8 billion years ago. atmosphere of the Earth.
- The term “second atmosphere” is a reference to a hypothetical atmospheric composition that is believed to have existed on Earth during its early geological history.
- The impact of life must be considered at an early stage in the history of the atmosphere, as indications of primitive life forms have been discovered dating back as far as 3.5 billion years ago.
- Throughout this particular era, the rocks emitted substantial amounts of gases, such as nitrogen, ammonia, carbon monoxide, and water vapor. This combination of gases is comparable to the emissions of contemporary volcanoes and fumaroles.
- Similar to contemporary volcanic emissions, it is hypothesized that the ancient atmosphere contained only minute amounts of oxygen, rendering it toxic to nearly all extant organisms.
Third Atmosphere
During the Phanerozoic Eon, which was characterized by the emergence of abundant animal life and the appearance of diverse hard-shelled animals, the Third Atmosphere came into existence. This period marked the emergence of oxygen-breathing metazoan life forms. The emergence of life, specifically in the form of “Archaea”, occurred approximately 3.5 billion years ago.
- Archaea represent a significant assemblage of unicellular entities lacking nuclei.
- Approximately 2.7 billion years in the past, cyanobacteria, a type of microbe, became integrated into the aforementioned group.
- Cyanobacteria, as the initial phototrophic organism capable of producing oxygen, gradually commenced the process of carbon dioxide absorption from the atmosphere and subsequent oxygen release.
The Modern Atmosphere
- The term “air” refers to the gaseous mixture that constitutes the Earth’s atmosphere and is utilized in respiration and the process of photosynthesis.
- According to scientific data, the composition of dry air in terms of volume is approximately 78.09% nitrogen, 20.95% oxygen, 0.93% argon, 0.039% carbon dioxide, and trace amounts of other gases such as neon, helium, ozone, and hydrogen.
- The atmosphere comprises a fluctuating quantity of water vapor, constituting 4% of its volume and 3% of its weight. An unfiltered air sample may contain a variety of natural substances in minuscule quantities, such as dust, pollen, salt, smoke and spores, sea spray, and volcanic ash.
- Several industrial pollutants, including chlorine, fluorine, elemental mercury, and sulfur compounds, may be detected.
- The atmospheric composition remains relatively stable up to an altitude of 50 km above the Earth’s surface, except for the presence of ozone and water vapor.
| Name of Stage | Duration of Stage (Billions of Years Ago) | Main Constituents of the Atmosphere | Features |
| Early Atmosphere | 4.4 to 4.0 | H2O, HCN, NH3, CH4, sulfur, iodine, bromine, chlorine, argon | Gases with lower molecular weights, such as hydrogen and helium, have been observed to have escaped into outer space. Due to elevated temperatures, all water was contained in the atmosphere in the form of vapor. |
| Secondary Atmosphere | 4.0 to 3.3 | At 4.0 billion H2O, CO2, and nitrogen (N) are dominant. Cooling of the atmosphere causes precipitation and the development of the oceans. By 3.0 billion H2O, CO2, N2 dominant. O2 begins to accumulate. | The persistent emission of gases from the lithosphere. Water vapor clouds are frequently observed in the lower atmosphere. Chemosynthetic bacteria emerged on the planet Earth during a period ranging from 3.9 to 3.5 billion years ago. The emergence of life leads to alterations in the composition of the atmosphere. |
| Modern Atmosphere | 3.3 to Present | N2 – 78%, O2 – 21%, Argon – 0.9%, CO2 – 0.036% | The proliferation, adaptation, and expansion of life forms have contributed to the augmentation of atmospheric oxygen levels. |
Theories of Evolution of Earth’s Atmosphere
Theories pertaining to the composition and formation of the Earth’s early atmosphere have undergone evolution and refinement over the course of history. The available evidence pertaining to the early atmosphere is restricted due to the vast time span of 4.6 billion years.
There exist several theories regarding the evolution of the atmosphere.
- According to a particular hypothesis, the initial billion years of Earth’s existence were characterized by significant volcanic eruptions that discharged gases, leading to the formation of the early atmosphere. This atmosphere primarily comprised carbon dioxide and lacked oxygen gas.
- The oceans were formed through the process of water vapor condensation.
- Volcanic activity is known to have generated nitrogen, which accumulated over time in the atmosphere, alongside minor quantities of methane and ammonia. The formation of oceans resulted in the dissolution of carbon dioxide in water, leading to the precipitation of carbonates and the consequent production of sediments. This process contributed to a decrease in the quantity of carbon dioxide present in the atmosphere.
Atmospheric Structure
Different classification schemes are used to designate various regions of the Earth’s atmosphere. The categorization systems utilized are founded upon factors such as temperature, composition, gravitation, and ionization.
Classification Based on Temperature (Thermal Structure)
The temperature classification of atmospheric layers is determined by the variances in temperature, which are primarily caused by the absorption of solar radiation. Specifically, the surface layer is affected by visible light, the middle layer is affected by near ultraviolet radiation, and the upper layer is affected by far ultraviolet radiation.
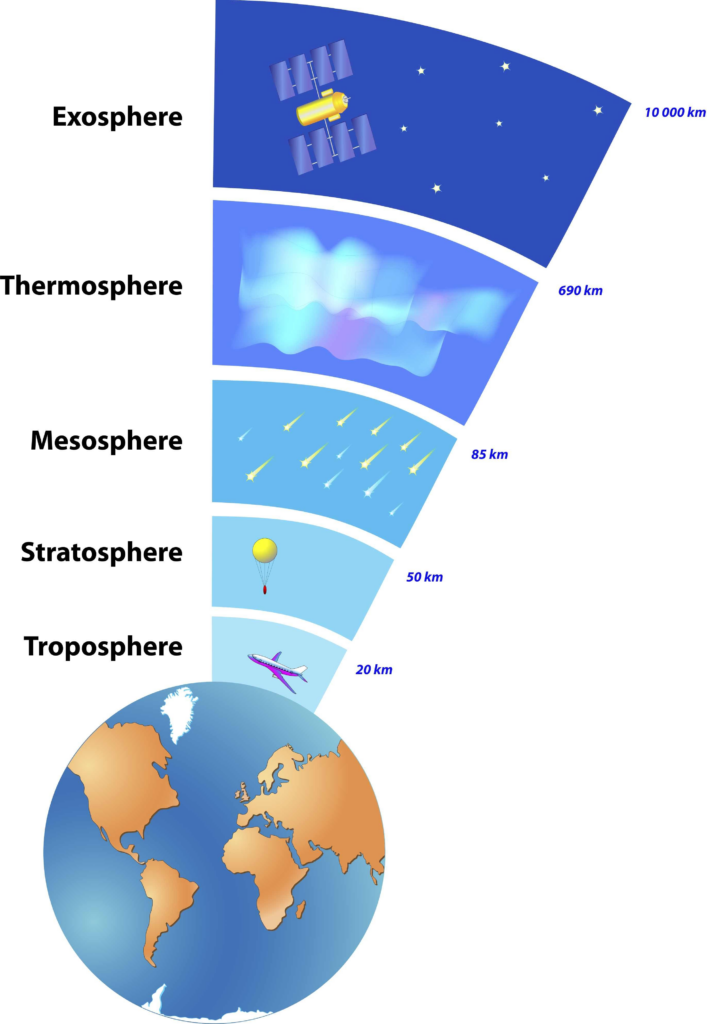
Troposphere
- The troposphere is the innermost layer of the Earth’s atmosphere and comprises approximately 80% of the total atmospheric mass.
- The troposphere experiences a rapid decrease in temperature and water vapor content as altitude increases.
- The regulation of air temperature is significantly influenced by water vapor due to its capacity to absorb solar energy and thermal radiation emanating from the planet’s surface.
- Approximately 99% of the atmospheric water vapor is found within the troposphere. The concentrations of water vapor exhibit variability across different latitudes.
- The highest concentrations of these entities are observed in regions located above the tropics, with elevations reaching up to 3%. As one moves towards the polar regions, the concentrations tend to decrease.
- It is within the confines of the troposphere that all weather phenomena take place, with the exception of turbulence which may have an impact on the lower region of the stratosphere. The term “troposphere” refers to the atmospheric layer characterized by intense convective air currents, earning it the moniker “region of mixing.”
Stratosphere
- The stratosphere represents the second significant layer of the Earth’s atmosphere.
- The temperature of the atmosphere in the stratosphere exhibits a state of relative constancy until it reaches an altitude of 25 kilometers. Subsequently, there is a gradual increment in altitude until reaching the stratopause. Due to the fact that the temperature of the air in the stratosphere rises as altitude increases, it does not induce convection and instead exerts a stabilizing impact on atmospheric conditions within the area.
- The regulation of the thermal regime of the stratosphere is primarily attributed to ozone, given the significantly low levels of water vapor content within this layer. There is a positive correlation between ozone concentration and temperature.
- The process of converting solar energy into kinetic energy occurs when ozone molecules assimilate ultraviolet radiation, leading to the warming of the stratosphere.
- The ozone layer is situated at an elevation ranging from 15 to 25 kilometers. Around 90% of the ozone present in the Earth’s atmosphere is located within the stratospheric layer. The concentration of ozone in this particular region is roughly 10 parts per million by volume (ppmv), which stands in stark contrast to the troposphere’s concentration of approximately 0.04 ppmv.
- The majority of solar ultraviolet radiation within the range of 290 nm – 320 nm (UV-B radiation) is absorbed by ozone. The absorption of these wavelengths by the nucleic acid in cells renders them harmful to life. The escalation in the infiltration of ultraviolet radiation onto the surface of the planet could result in detrimental environmental outcomes and harm the vegetation. Significant quantities of solar ultraviolet radiation could lead to various biological consequences, including a substantial rise in cancer incidence.
Mesosphere
- The mesosphere is a stratum that spans an altitude range of roughly 50 to 85 kilometers above the Earth’s surface. This layer is distinguished by a decline in temperature.
- The mesopause, situated at the uppermost layer of Earth’s atmosphere, is known to exhibit the most frigid temperatures, particularly during summer in the polar regions.
- The mesosphere has been colloquially dubbed the “ionosphere” due to its relatively limited research compared to other atmospheric layers.
- The atmospheric region encompassing the stratosphere and mesosphere is commonly denoted as the middle atmosphere.
Thermosphere
- The thermosphere is situated at an altitude higher than that of the mesosphere.
- The temperature gradient in the thermosphere exhibits a positive correlation with altitude, culminating in a range of 600-2000 K, contingent upon the level of solar activity.
- The rise in temperature can be attributed to the absorption of concentrated solar radiation by the restricted quantity of residual molecular oxygen. At high elevations, the distance between gas molecules is significantly increased.
- At an altitude exceeding 100 km from the Earth’s surface, the chemical composition of the atmosphere exhibits a significant dependence on altitude, resulting in an enrichment of lighter gases such as atomic oxygen, helium, and hydrogen.
Exosphere
- The exosphere denotes the outermost layer of the Earth’s atmosphere, situated at the farthest distance from the planet’s surface.
- The lower boundary of the atmosphere, referred to as the thermopause or exobase, exhibits an altitude range of approximately 250-500 km, which is contingent upon the level of solar activity. The theoretical definition of the upper boundary is based on the altitude, approximately 193,000 km, which is equivalent to half the distance to the Moon. At this altitude, the impact of solar radiation pressure on atomic hydrogen velocities surpasses that of the Earth’s gravitational pull.
- The exosphere, commonly referred to as the geocorona, has been observed from space and has been found to have an extension of at least 97,000 km from the Earth’s surface. The exosphere can be defined as the intermediary region that separates the Earth’s atmosphere from the vast expanse of interplanetary space.
Classification Based on Ions (Magneto-electronic structure)
The uppermost layer of the Earth’s atmosphere is stratified into distinct regions that are delineated by the conductive properties and abundance of unbound electrons and other ionized particles.
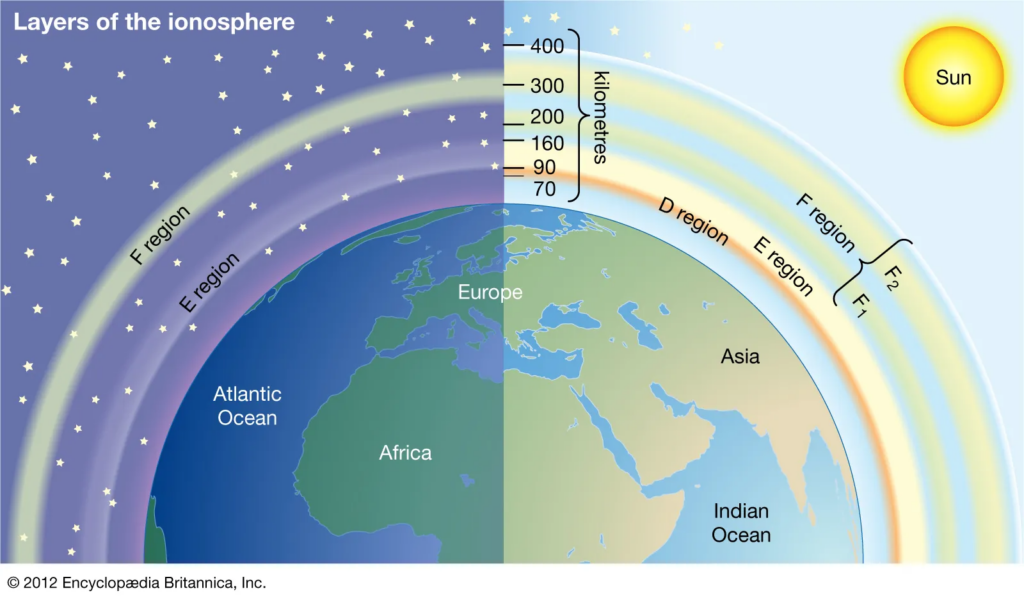
Ionosphere
- The ionosphere is a layer of the Earth’s atmosphere that is located approximately 60 to 1,000 kilometers above the surface. It is characterized by a high concentration of ions and free electrons, which are created by the ionization of atmospheric gases by solar radiation.
- This layer plays an important role in radio communication and navigation, as it reflects radio waves back to the Earth’s surface. The ionosphere is divided into several layers, including the D, E, and F layers, which vary in altitude and ionization levels.
- The ionosphere is characterized by the impact of atmospheric phenomena on the transmission of radiowaves, which is caused by the existence and fluctuations in the abundance of unbound electrons within the atmosphere.
- The D-region is situated at an altitude ranging from 60 to 90 kilometers, and it undergoes a process of disappearance during the night.
- The E-region is situated at an altitude ranging from 90 to 140 kilometers.
- The F-region is situated at an altitude exceeding 140 kilometers. Throughout the diurnal cycle, the ionosphere exhibits two distinct regions, namely the F1-region spanning an altitude range of approximately 140 to 180 km and the F2-region characterized by a peak electron concentration within the altitude range of approximately 250 to 500 km.
- The region of the ionosphere characterized by the highest concentration of electrons is commonly known as the Topside Ionosphere.
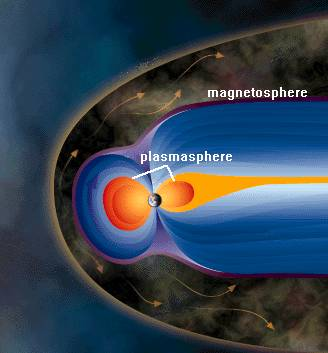
Plasmasphere
- The plasmasphere, a region surrounding the Earth, exhibits a toroidal shape rather than a perfect sphere, with the central opening aligned along the planet’s magnetic axis.
- The plasmasphere of the Earth is composed solely of plasma, which is recognized as the fourth fundamental state of matter.
- The plasma under consideration is primarily constituted of hydrogen ions, also known as protons, and electrons.
- The plasmapause is characterized by a highly defined and acute boundary. Typically, the periphery of this toroidal-shaped pastry situated along the equatorial plane is positioned at an altitude ranging from 19,000 to 32,000 kilometers above the planetary surface.
- The plasmasphere can be regarded as an extension of the ionosphere. Within the plasmapause region, the geomagnetic field lines undergo rotational motion in tandem with the Earth’s rotation.
- The altitude at which protons supersede oxygen as the primary species in the ionospheric plasma is commonly regarded as the inner boundary of the plasmasphere, typically occurring at an altitude of approximately 1000 km. The plasmasphere may be regarded as a constituent element of the magnetosphere.
Magnetosphere
- Beyond the plasmapause region, the rotation of magnetic field lines is impeded due to the significant impact of electric fields originating from the solar wind.
- The magnetosphere is a non-spherical cavity that confines the Earth’s magnetic field through the influence of the solar wind and interplanetary magnetic field (IMF).
- The region demarcating the outermost extent of the magnetosphere is referred to as the magnetopause.
- The configuration of the magnetosphere resembles that of an elongated teardrop, akin to the shape of a Christmas Tree ornament, with its tail oriented in the direction opposite to that of the Sun.
- The magnetopause is situated approximately 56,000 km above the Earth’s surface on the diurnal side and extends into a lengthy magnetotail, measuring several million kilometers in length (roughly 1000 Earth radii), extending well beyond the Moon’s orbit (at approximately 60 Earth radii) on the nocturnal side of the Earth. Nevertheless, it is typical for the Moon to remain outside of the magnetosphere, with the exception of a brief period of approximately two days surrounding the occurrence of a Full Moon.
- The magnetosheath and bow shock are areas within the solar wind that have been perturbed by the Earth’s magnetic field and its presence beyond the magnetopause.
Composition of Earth’s Atmosphere
In terms of relative abundance, the atmospheric composition of Earth is comprised of nitrogen, oxygen, argon, carbon dioxide, and a variety of trace gases. Due to significant geographical variability, the total amount does not include water vapor.
Nitrogen
- Although nitrogen is the most prevalent gas in the Earth’s atmosphere, it constitutes merely 0.005% of the Earth’s crust by weight, as per David Darling’s research.
- The element nitrogen exhibits remarkable stability and necessitates a substantial amount of energy to undergo any alterations in its chemical configuration.
- Despite its relatively small volume in the Earth’s crust, nitrogen assumes a significant function in the nitrogen cycle.
- Within this particular biological process, nitrogen undergoes a perpetual exchange between the Earth’s atmosphere and various living organisms.
Oxygen
- The planet Earth possesses the necessary environmental conditions to support and promote the thriving of living organisms. The inhalation of oxygen is a vital component of human existence, as it is utilized in the process of respiration within the lungs and subsequently contributes to metabolic processes.
- Although nitrogen is a highly stable gas, its utilization for chemical reactions poses a challenge due to its resistance to fragmentation. Oxygen exhibits a high propensity to engage in chemical reactions due to its electron-withdrawing nature.
- Despite the abundance of nitrogen, the presence of oxygen is essential for facilitating chemical reactions that generate energy.
Argon
- Argon, being an inert gas, exhibits minimal reactivity and does not readily form chemical bonds or participate in atmospheric processes.
- The absence of an argon cycle can be attributed to the following reasons. Nitrogen and carbon are present due to their capacity to form chemical bonds with other elements.
- One of the potential outcomes of the radioactive decay of potassium is the production of argon. The lithosphere is known to contain a significant amount of potassium.
- It is present in the atmosphere at a concentration of 0.93% by volume.
Carbon dioxide (CO2)
- Carbon is considered the preeminent element in the construction of molecules that are fundamental to the existence of living organisms.
- As evidenced by the long-term carbon cycle, carbon exists in diverse forms, including carbon dioxide (CO2), methane (CH4), and glucose (C6H12O6).
- The rise in carbon dioxide levels since 1900 can be primarily attributed to anthropogenic factors. Following the extraction of fossil fuels, individuals engage in the combustion of said fuels.
- Methane and carbon dioxide are transformed into atmospheric pollutants. The concentration of carbon dioxide has increased by almost two-fold since the year 1900.
Trace Gases
- The residual fraction of the atmosphere is comprised of trace gases. Several minor trace gases, such as neon, helium, methane, and krypton, constitute a small fraction of the atmosphere.
- However, it is noteworthy that humans are also capable of contributing to the emission of certain trace gases. The ozone layer in the polar regions has been adversely affected by chlorofluorocarbons (CFCs).
- Upon entering the troposphere and subsequently the stratosphere, chlorine undergoes a reaction with ozone (O3), resulting in its depletion. Water vapor, akin to ozone, is a gas that exhibits variability.
| Nitrogen | 78.08% |
| Oxygen | 20.95% |
| Argon | 0.93% |
| Carbon dioxide | 0.03% |
Changes in Atmosphere
- The volcanic activity is characterized by a high degree of intensity, resulting in the release of various gases such as water vapour, carbon dioxide, nitrogen, ammonia, and methane.
- The process of water vapor condensation resulted in the formation of oceans.
- The composition of the atmosphere is primarily carbon dioxide, with a gradual accumulation of nitrogen over a period of time.
- The process of carbon dioxide dissolution in oceans and subsequent precipitation of carbonates leading to the formation of sediments is observed. The concentration of carbon dioxide in the Earth’s atmosphere is decreasing.
- Algae engage in the process of photosynthesis, resulting in the production of oxygen.
- The evolution of plants led to a reduction in carbon dioxide levels and an increase in oxygen levels, which in turn facilitated the evolution of animals.
- The process of sedimentary rock formation and fossil fuel generation resulted in a reduction in carbon dioxide levels, as a consequence of the incorporation of sediments and organic matter, respectively.
References
- https://www.britannica.com/science/ionosphere-and-magnetosphere/Magnetosphere
- https://homapilot.com/articles/ionosphere/
- https://overallscience.com/atmosphere-introduction-and-structure/
- https://earthhow.com/earth-atmosphere-composition/
- https://byjus.com/free-ias-prep/ncert-notes-structure-of-atmosphere/#:~:text=The%20lower%20Thermosphere%20is%20called,400%20km%20above%20the%20Mesopause.
- https://education.nationalgeographic.org/resource/atmosphere/
- https://studymind.co.uk/notes/changes-in-the-earths-atmosphere/
- Regents of the University of Michigan, Evolution Of The Atmosphere: Composition, Structure And Energy, 2022.
- Revise Chemistry, AQA GCSE Chemistry of the Atmosphere, 2020.
- John Grotzinger, Understanding Earth (International Edition), 2019.
- https://www.visionlearning.com/en/library/Earth-Science/6/Composition-of-Earths-Atmosphere/107
- https://uomustansiriyah.edu.iq/media/lectures/6/6_2018_10_09!11_41_38_AM.pdf
- Jon Shonk, Introducing Meteorology: A Guide to Weather, 2013.
