If the chemical equilibrium is the result of two active and opposing reactions, the system’s equilibrium is referred to as a dynamic equilibrium. Dynamic equilibria occur when there is no observable change in a reaction, but the system is still constantly in motion, with reactants being continuously converted into products and products being continuously converted into reactants. The system is considered a closed system (sealed container), which means that no components are added or removed from it.
Definition of Dynamic Equilibrium
A dynamic equilibrium is the state of a reversible reaction in which the forward reaction rate equals the backward reaction rate and the reactant and product concentrations remain constant. This movement happens at the same rate, and there is no net change in the reactant/product ratio.
A system in a steady state is an example of dynamic equilibrium. This means that the variables in the equation remain constant over time (since the rates of reaction are equal). A reaction in dynamic equilibrium appears to be inactive because the concentrations of each substance remain constant. However, reactions are constantly taking place.
Chemical reactions can move either forward or backward or or they can only move in one direction. Reversible reactions are those that go in two directions and can be identified by the arrows going in two directions, as shown in the example below.
H2O (l) ⇌ H+ (aq) + OH– (aq)
Only reversible reactions can reach dynamic equilibrium, and that only happens when the forward reaction’s rate is equal to the reverse reaction’s rate. These equations are dynamic because the forward and reverse reactions continue to occur, but the two rates are equal and constant, indicating that they are also at equilibrium.
Dynamic Equilibrium Examples
- Acetic acid dissociates in a single-phase system, resulting in an acid-base equilibrium. The following reaction describes this state of dynamic equilibrium.
CH3COOH ⇌ CH3COO– + H+
- The dimerization of nitrogen dioxide in the gaseous phase is a good example of dynamic equilibrium.
2NO2 ⇌ N2O4
- Industrial ammonia synthesis using Haber’s process.
N2 (g) + 3H2 (g) ⇌ 2NH3 (g)
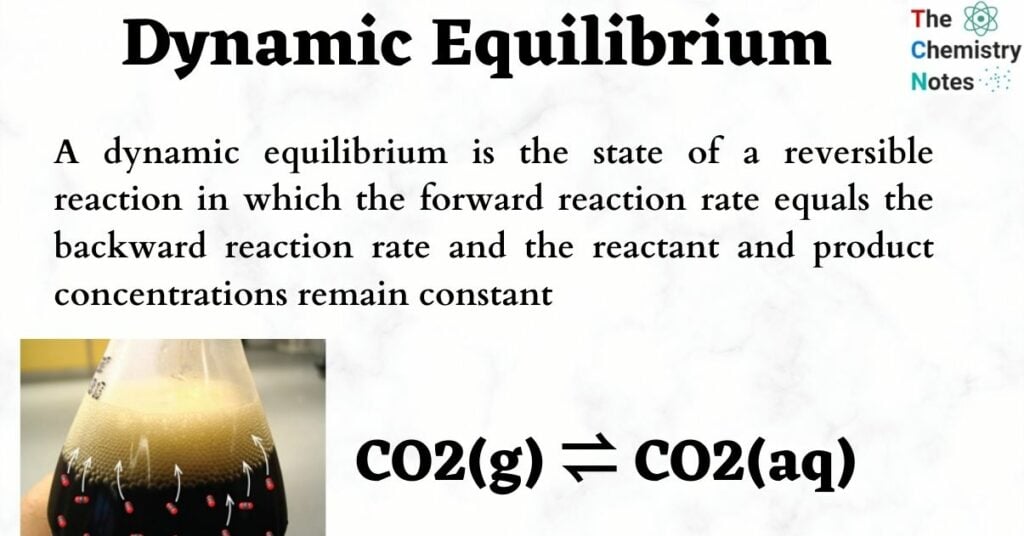
- Each time you’ve had a soda, you’ve seen an illustration of dynamic equilibrium. Carbon dioxide is present in both the liquid/aqueous and gaseous phases of a sealed bottle of soda (bubbles). The two phases of carbon dioxide are in dynamic equilibrium inside the sealed soda bottle because gaseous carbon dioxide dissolves into liquid carbon dioxide at the same rate that liquid carbon dioxide is converted back to gaseous carbon dioxide.
CO2(g) ⇌ CO2(aq)
The equilibrium concentration of carbon dioxide in the liquid phase is proportional to the partial pressure of the CO2 gas in the bottle, demonstrating Henry’s Law.
An equation’s equilibrium can be altered by altering the reaction’s temperature, pressure, or concentration, which can cause the equation to lose its dynamic equilibrium. This is why, if you open a soda can and leave it out for a long time, it will eventually become “flat,” with no more bubbles. Because the soda can is no longer a closed system, the carbon dioxide can interact with the surrounding environment. This knocks it out of dynamic equilibrium and causes it to emit gaseous carbon dioxide until no more bubbles form.
- Assume you prepare a solution saturated with an aqueous NaCl solution. If you then add solid crystals of NaCl to the solution, the NaCl will dissolve and recrystallize at the same time.
NaCl (s) ⇌ Na+(aq) + Cl–(aq)
The reaction will be in dynamic equilibrium when the rate of NaCl dissolution equals the rate of recrystallization.
- Nitrogen dioxide (NO2) reacts with carbon monoxide (CO) to form nitrogen oxide (NO) and carbon dioxide (CO2), and nitrogen oxide and carbon dioxide react to form nitrogen dioxide and carbon monoxide in the reverse reaction.
NO2(g) + CO(g) ⇌ NO(g) + CO2(g)
This is another example of dynamic equilibrium (again, as long as the two rates are equal).
Production of Ammonia and Equilibrium
The Haber process is used in the synthesis of ammonia.
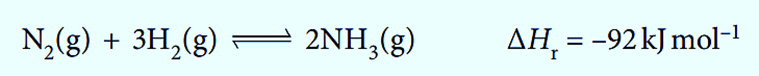
We can use Le Chatelier’s principle to show how to get the highest ammonia yield. When the reaction is faster at high temperatures, the position of equilibrium is to the left because the reaction is exothermic (ΔH is negative).
What happens if we apply more pressure?
- When we increase the pressure, the reaction changes, resulting in fewer molecules of gas being formed.
- The equilibrium shifts in the direction of lower pressure.
- In this case, there are four gas molecules on the left side and two on the right. As a result, the balance shifts to the right.
- Ammonia production rises.
What happens if we lower the temperature?
- A decrease in temperature reduces the energy of the environment.
- The reaction will proceed in the direction of energy release.
- The exothermic reaction releases energy, and the equilibrium position favors ammonia production.
- This shifts the equilibrium position to the right. The Value of Kp rises.
What happens if we condense ammonia to a liquid to remove it?
ammonia has a much higher boiling point than hydrogen and nitrogen, thus we are able to remove it by condensing.
- To replace the ammonia that has been removed, the position of equilibrium shifts to the right.
- In order to keep the value of Kp constant, more ammonia is formed from hydrogen and nitrogen.
Differences Between Static and Dynamic Equilibrium
No clear changes are observed between static and dynamic equilibrium. However, reactions in static equilibrium differ significantly from those in dynamic equilibrium.
When a reaction reaches static equilibrium (also known as mechanical equilibrium), there is no movement between the reactants and products. The reaction is finished, and both the forward and reverse reaction rates are zero.
While reactions in dynamic equilibrium are reversible (they can go either way), those in static equilibrium are irreversible and can only go one way. However, both dynamic equilibrium and static equilibrium are examples of steady-state systems in which the net force action on the system is constant.
Have a look at this video for more clear information about dynamic equilibrium. https://youtu.be/wlD_ImYQAgQ
References
- Atkins, P.W.; de Paula, J. (2006). Physical Chemistry (8th. ed.). Oxford University Press. ISBN 0-19-870072-5.
- Denbeigh, K (1981). The principles of chemical equilibrium (4th. ed.). Cambridge, U.K.: Cambridge University Press. ISBN 0-521-28150-4.
- https://byjus.com/jee/dynamic-equilibrium/
- https://blog.prepscholar.com/what-is-dynamic-equilibrium-definition-example
- https://www.thoughtco.com/definition-of-dynamic-equilibrium-605052

