Elements undergo chemical reactions to acquire a full outer shell of electrons, which will lead to a more stable energy state. This is accomplished through chemical bonding in which old bonds are broken and new bonds are formed. Thermal energy is transferred into and out of reaction mixtures during this process. The energy in the system comes from the chemical bonds, which can be thought of as tiny stores of chemical energy.
Many chemical reactions produce heat, light, or sound as a by-product. This is an exothermic reaction. Exothermic reactions in the lab generate heat and may even be explosive.
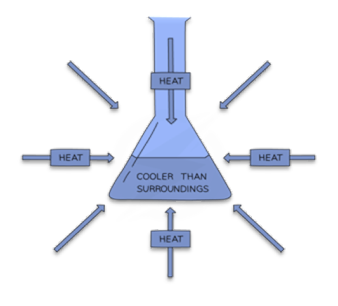
Other chemical reactions, in order to proceed, must absorb energy. Endothermic reactions occur. Endothermic reactions cannot occur on their own. Work is required in order for these reactions to occur. A temperature drop is measured during endothermic reactions that absorb energy.
Energy is released during the formation of the end products (sodium acetate, water, and carbon dioxide) as attracted atoms are brought back together. Some bonds between atoms in the reactants breaks during a chemical reaction, but not all.
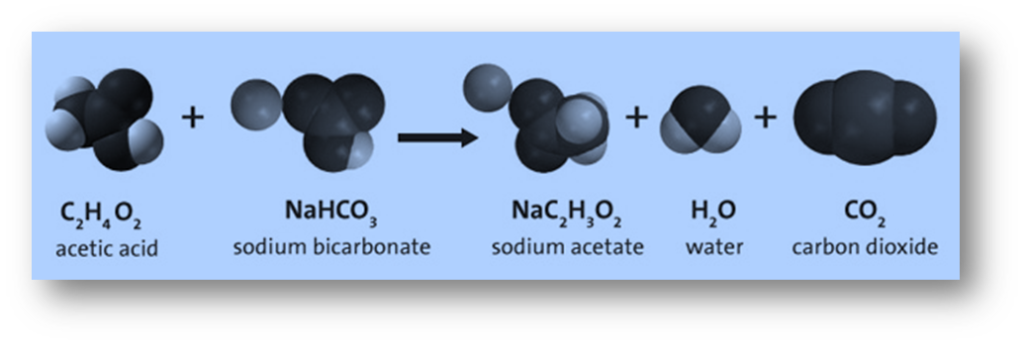
You can determine whether a chemical reaction releases or absorbs energy overall by comparing the energy used when bonds in the reactants are broken with the energy released when bonds in the products are formed.
Endothermic Processes
The term “endothermic process” refers to a reaction in which the system takes up heat from its environment.
Endothermic processes include photosynthesis, evaporating liquids, melting ice, dry ice, alkanes cracking, thermal decomposition, ammonium chloride in water, and many others.
Endo means “to absorb,” and thermic means “heat,” as the name suggests.
- The conversion of this energy from the reactants into the product occurs during the reaction of the reactants. The dissociation of the molecules’ bonds is what causes it to happen. The energy releases as new bonds forms.
- As a result of these reactions, which absorb heat from their surroundings, the system’s temperature stays lower. In addition, the enthalpy, which is the change in heat energy during the conversion of reactants to products, increases at the end of the reaction.
- The energy of the system increases as this energy is transferred to the chemical energy store, indicating a positive energy change.
- The overall transfer of heat occurs from the environment to the system.
- These reactions are far less common than exothermic reactions.
- Endothermic reactions include electrolysis, thermal decomposition reactions, and the first stages of photosynthesis.
Examples of Endothermic Reactions
- Endothermic chemical reactions include photosynthesis. Plants use the energy from the sun to convert carbon dioxide and water into glucose and oxygen in this process. For every kilogram of glucose produced, this reaction requires 15MJ of energy (sunlight):
sunlight + 6CO2 (g) + H2O (l) → C6H12O6 (aq) + 6O2 (g)
- Dissolving ammonium chloride in water.
NH4Cl (s) + H2O (l) → NH4+ (aq) + Cl− (aq) ΔH=15.15kJ/mol
- Cracking alkane is an endothermic reaction.
- NaCl dissolution: Endothermic dissolution of sodium chloride, or common table salt, occurs. That is, if you dissolve it in water, the water will become slightly colder as a result of the energy absorbed by the ion solvation.
NaCl → Na+ (aq) + Cl– (aq)
However, this is not necessarily true for all salts. Depending on the substance dissolved, dissolution can be both endothermic and exothermic!
- Ethanoic acid reacts with sodium carbonate as follows:
CH3COOH (aq) + Na2CO3 (s) + heat → CH3COO–Na+ (aq) + H+ (aq) + CO32- (aq)
- Mixing water with potassium chloride:
KCl (s) + H2O (l) + heat → KCl (aq)
Exothermic Processes
Exothermic reactions are the inverse of endothermic reactions which emits energy to their surroundings in the form of light or heat.
Examples include neutralization, burning something, fuel reactions, the deposition of dry ice, respiration, the solution of sulfuric acid in the water, and many more.
The terms ‘exo’ and ‘thermic’ mean ‘to release’ and ‘heat,’ respectively.
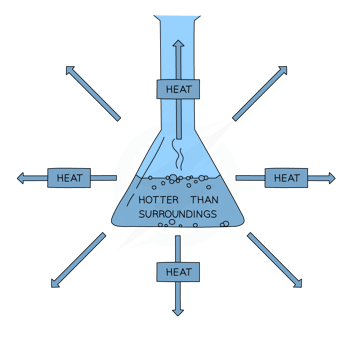
- Exothermic reactions involve the transfer of thermal energy to the environment, raising the ambient temperature.
- The energy released is the result of new bonds (products) formation at a higher level. While the amount of energy required to break up the bonds (reactants) is less.
- The system’s energy decreases as a result of the energy transformation from its chemical energy reserve to its surroundings, indicating a negative energy change.
- The enthalpy change decreases as the reaction progresses. A great deal of energy is necessary during chemical reactions.
- Hence, a tremendous amount of energy releases as a result of the reactions between molecules and compounds, as well as the breaking of bonds.
- Overall transfer occurs from the system to the environment.
- Reactions that are typically exothermic include combustion, oxidation, and neutralization.
Examples of Exothermic Reactions
- Combustion is the process by which a substance burns in the presence of oxygen, emitting heat and light. These reactions produce a lot of energy in the form of heat, but they also produce some byproducts like smoke.
CH4 + O2 → CO2 + H2O + Heat
- Humans absorb oxygen through respiration and exhale carbon dioxide through the same process. It is worth noting that C6H12O6 is the formula for glucose, which combines with the oxygen we breathe to form carbon dioxide, water, and energy.
C6H12O6 + 6O2 → 6CO2 + 6H2O + Energy
- It is an exothermic reaction. Preparation of slaked lime is also an example for exothermic reaction.
CaO + H2O → Ca(OH)2 + heat
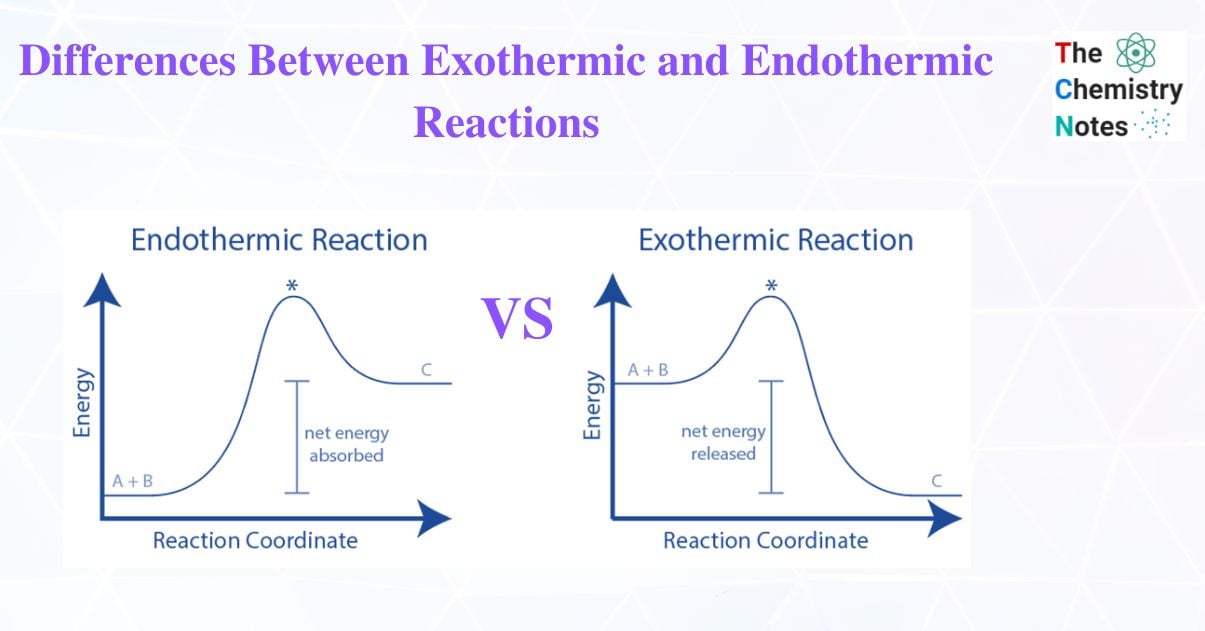
Energy Level Diagram of Endothermic and Exothermic Reactions
Graphical representations of the relative energies of the reactants and products in chemical reactions are reaction pathway diagrams.
The height difference between reactant and product energy represents the overall energy change of a reaction.
Arrows on the diagrams indicate whether the reaction is exothermic (the overall reaction arrow is pointing downward, indicating that the system has lost energy) or endothermic (the system has gained energy) (overall reaction arrow is upwards pointing, showing that the system has gained energy)
The activation energy is the initial increase in energy (Ea), which is the minimum energy requirement for colliding particles to react.
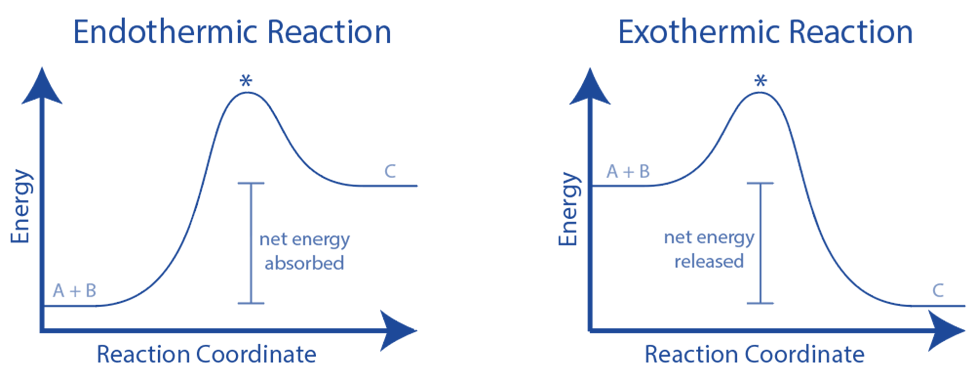
A reaction pathway diagram for an exothermic reaction explains the product’s energy is lower than the reactants’. (Here the thermal energy is transfer to the surroundings). A reaction pathway diagram for an endothermic reaction explains the energy of the product is greater than the energy of the reactants. (Thus, the thermal energy has been taken in from the surroundings)
Difference Between Endothermic and Exothermic Reactions
| Endothermic Reaction | Exothermic Reaction |
| Endothermic reactions are chemical reactions that produce products by absorbing heat energy from their surroundings. | An exothermic reaction is one that releases energy in the form of light or heat. |
| The reaction absorbs energy from its surroundings. | The energy is released from the system to its environment. |
| Energy in the form of heat | Energy is released as heat, electricity, light or sound. |
| Enthalpy is positive, as heat is absorbed. | Enthalpy is negative, as heat is evolved. |
| The temperature drops as endothermic reactions progress. | The temperature rises as exothermic reactions progress. |
| The change in enthalpy (ΔH) is positive. | The change in enthalpy (ΔH) is negative. |
| Melting ice, evaporation, cooking, gas molecules, photosynthesis are a few examples | Rusting iron, settling, chemical bonds, explosions, nuclear fission are a few examples. |
| NH4Cl (s) + H2O (l) + heat → NH4Cl (aq) | 2H2 (g) + O2 (g) → 2H2O (l) + heat |
References
- R. Chang, “Physical Chemistry for the Chemical and Biological Sciences”, University Science Books, Sausalito, California (2000).
- Aston, J. and Fritz, J.J., Thermodynamics and Statistical Mechanics, John Wiley and Sons, Inc., New York, 1959.
- https://byjus.com/ chemistry/ endothermic-exothermic-reactions-difference/
- https://www.thoughtco.com/endothermic-and-exothermic-reactions-602105
